The SCN8A encephalopathy mutation p.Ile1327Val displays elevated sensitivity to the anticonvulsant phenytoin
- PMID: 27375106
- PMCID: PMC5012949
- DOI: 10.1111/epi.13461
The SCN8A encephalopathy mutation p.Ile1327Val displays elevated sensitivity to the anticonvulsant phenytoin
Abstract
Objective: SCN8A encephalopathy (early infantile epileptic encephalopathy; EIEE13) is caused by gain-of-function mutations resulting in hyperactivity of the voltage-gated sodium channel Nav 1.6. The channel is concentrated at the axon initial segment (AIS) and is involved in establishing neuronal excitability. Clinical features of SCN8A encephalopathy include seizure onset between 0 and 18 months of age, intellectual disability, and developmental delay. Seizures are often refractory to treatment with standard antiepileptic drugs, and sudden unexpected death in epilepsy (SUDEP) has been reported in approximately 10% of patients. In a recent study, high doses of phenytoin were effective in four patients with SCN8A encephalopathy. In view of this observation, we have investigated the relationship between the functional effect of the SCN8A mutation p.Ile1327Val and its response to phenytoin.
Methods: The mutation was introduced into the Scn8a cDNA by site-directed mutagenesis. Channel activity was characterized in transfected ND7/23 cells. The effects of phenytoin (100 μm) on mutant and wild-type (WT) channels were compared.
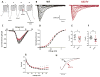
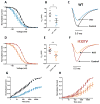
Results: Channel activation parameters were shifted in a hyperpolarizing direction in the mutant channel, whereas inactivation parameters were shifted in a depolarizing direction, increasing Na channel window current. Macroscopic current decay was slowed in I1327V channels, indicating an impairment in the transition from open state to inactivated state. Channel deactivation was also delayed, allowing more channels to remain in the open state. Phenytoin (100 μm) resulted in hyperpolarized activation and inactivation curves as well as greater tonic block and use-dependent block of I1327V mutant channels relative to WT.
Significance: SCN8A - I1327V is a gain-of-function mutation with altered features that are predicted to increase neuronal excitability and seizure susceptibility. Phenytoin is an effective inhibitor of the mutant channel and may be of use in treating patients with gain-of-function mutations of SCN8A.
Keywords: Anticonvulsant drugs; Epileptic encephalopathy; Phenytoin; SCN8A; Sodium channels.
Wiley Periodicals, Inc. © 2016 International League Against Epilepsy.
Conflict of interest statement
of Conflicts of Interest None of the authors has any conflict of interest.
Figures
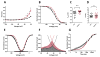

References
-
- Vreugdenhil M, Hoogland G, van Veelen CW, et al. Persistent sodium current in subicular neurons isolated from patients with temporal lobe epilepsy. Eur J Neurosci. 2004;19:2769–2778. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

