Biosynthetic Polymers as Functional Materials
- PMID: 27375299
- PMCID: PMC4928144
- DOI: 10.1021/acs.macromol.6b00439
Biosynthetic Polymers as Functional Materials
Abstract
The synthesis of functional polymers encoded with biomolecules has been an extensive area of research for decades. As such, a diverse toolbox of polymerization techniques and bioconjugation methods has been developed. The greatest impact of this work has been in biomedicine and biotechnology, where fully synthetic and naturally derived biomolecules are used cooperatively. Despite significant improvements in biocompatible and functionally diverse polymers, our success in the field is constrained by recognized limitations in polymer architecture control, structural dynamics, and biostabilization. This Perspective discusses the current status of functional biosynthetic polymers and highlights innovative strategies reported within the past five years that have made great strides in overcoming the aforementioned barriers.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


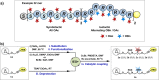



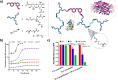
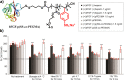
References
-
- Günay K. A.; Theato P.; Klok H.-A.. History of Post-Polymerization Modification. In Functional Polymers by Post-Polymerization Modification; Wiley-VCH Verlag GmbH & Co. KGaA: 2012; pp 1–44.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
