Carbon Nanomaterials Interfacing with Neurons: An In vivo Perspective
- PMID: 27375413
- PMCID: PMC4899452
- DOI: 10.3389/fnins.2016.00250
Carbon Nanomaterials Interfacing with Neurons: An In vivo Perspective
Abstract
Developing new tools that outperform current state of the art technologies for imaging, drug delivery or electrical sensing in neuronal tissues is one of the great challenges in neurosciences. Investigations into the potential use of carbon nanomaterials for such applications started about two decades ago. Since then, numerous in vitro studies have examined interactions between these nanomaterials and neurons, either by evaluating their compatibility, as vectors for drug delivery, or for their potential use in electric activity sensing and manipulation. The results obtained indicate that carbon nanomaterials may be suitable for medical therapies. However, a relatively small number of in vivo studies have been carried out to date. In order to facilitate the transformation of carbon nanomaterial into practical neurobiomedical applications, it is essential to identify and highlight in the existing literature the strengths and weakness that different carbon nanomaterials have displayed when probed in vivo. Unfortunately the current literature is sometimes sparse and confusing. To offer a clearer picture of the in vivo studies on carbon nanomaterials in the central nervous system, we provide a systematic and critical review. Hereby we identify properties and behavior of carbon nanomaterials in vivo inside the neural tissues, and we examine key achievements and potentially problematic toxicological issues.
Keywords: carbon nanomaterials; central nervous system; drug delivery; imaging; in vivo studies; neuroprotection.
Figures

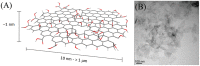
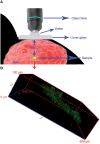
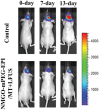


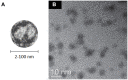
Similar articles
-
The interplay between carbon nanomaterials and amyloid fibrils in bio-nanotechnology.Nanoscale. 2013 Jul 21;5(14):6207-18. doi: 10.1039/c3nr01644g. Epub 2013 Jun 6. Nanoscale. 2013. PMID: 23744243 Review.
-
Theranostic applications of carbon nanomaterials in cancer: Focus on imaging and cargo delivery.J Control Release. 2015 Jul 28;210:230-45. doi: 10.1016/j.jconrel.2015.04.021. Epub 2015 Apr 21. J Control Release. 2015. PMID: 25910580 Review.
-
Carbon nanomaterials for drug delivery and tissue engineering.Front Chem. 2022 Sep 12;10:990362. doi: 10.3389/fchem.2022.990362. eCollection 2022. Front Chem. 2022. PMID: 36171994 Free PMC article. Review.
-
Functional DNA-containing nanomaterials: cellular applications in biosensing, imaging, and targeted therapy.Acc Chem Res. 2014 Jun 17;47(6):1891-901. doi: 10.1021/ar500078f. Epub 2014 Apr 29. Acc Chem Res. 2014. PMID: 24780000 Free PMC article.
-
Zero-Dimensional Carbon Nanomaterials for Fluorescent Sensing and Imaging.Chem Rev. 2023 Sep 27;123(18):11047-11136. doi: 10.1021/acs.chemrev.3c00186. Epub 2023 Sep 7. Chem Rev. 2023. PMID: 37677071 Review.
Cited by
-
Comprehensive Survey on Nanobiomaterials for Bone Tissue Engineering Applications.Nanomaterials (Basel). 2020 Oct 13;10(10):2019. doi: 10.3390/nano10102019. Nanomaterials (Basel). 2020. PMID: 33066127 Free PMC article. Review.
-
Carbon fibres as potential bone implants with controlled doxorubicin release.Sci Rep. 2022 Feb 16;12(1):2607. doi: 10.1038/s41598-022-06044-7. Sci Rep. 2022. PMID: 35173195 Free PMC article.
-
Inorganic Nanomaterial for Biomedical Imaging of Brain Diseases.Molecules. 2021 Dec 3;26(23):7340. doi: 10.3390/molecules26237340. Molecules. 2021. PMID: 34885919 Free PMC article. Review.
-
Crossing the blood-brain barrier with carbon dots: uptake mechanism and in vivo cargo delivery.Nanoscale Adv. 2021 May 31;3(13):3942-3953. doi: 10.1039/d1na00145k. eCollection 2021 Jun 30. Nanoscale Adv. 2021. PMID: 34263140 Free PMC article.
-
Soft Devices for High-Resolution Neuro-Stimulation: The Interplay Between Low-Rigidity and Resolution.Front Med Technol. 2021 Jun 14;3:675744. doi: 10.3389/fmedt.2021.675744. eCollection 2021. Front Med Technol. 2021. PMID: 35047928 Free PMC article. Review.
References
-
- Adelene Nisha J., Yudasaka M., Bandow S., Kokai F., Takahashi K., Iijima S. (2000). Adsorption and catalytic properties of single-wall carbon nanohorns. Chem. Phys. Lett. 328, 381–386. 10.1016/S0009-2614(00)00956-8 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

