Shielded Coaxial Optrode Arrays for Neurophysiology
- PMID: 27375415
- PMCID: PMC4899445
- DOI: 10.3389/fnins.2016.00252
Shielded Coaxial Optrode Arrays for Neurophysiology
Abstract
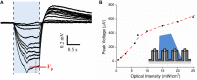
Recent progress in the study of the brain has been greatly facilitated by the development of new tools capable of minimally-invasive, robust coupling to neuronal assemblies. Two prominent examples are the microelectrode array (MEA), which enables electrical signals from large numbers of neurons to be detected and spatiotemporally correlated, and optogenetics, which enables the electrical activity of cells to be controlled with light. In the former case, high spatial density is desirable but, as electrode arrays evolve toward higher density and thus smaller pitch, electrical crosstalk increases. In the latter, finer control over light input is desirable, to enable improved studies of neuroelectronic pathways emanating from specific cell stimulation. Here, we introduce a coaxial electrode architecture that is uniquely suited to address these issues, as it can simultaneously be utilized as an optical waveguide and a shielded electrode in dense arrays. Using optogenetically-transfected cells on a coaxial MEA, we demonstrate the utility of the architecture by recording cellular currents evoked from optical stimulation. We also show the capability for network recording by radiating an area of seven individually-addressed coaxial electrode regions with cultured cells covering a section of the extent.
Keywords: extracellular; multielectrode array; nanotechnology; neuroelectronic; optogenetics; optrode.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

