Peptidylarginine Deiminase Inhibitor Suppresses Neutrophil Extracellular Trap Formation and MPO-ANCA Production
- PMID: 27375623
- PMCID: PMC4896908
- DOI: 10.3389/fimmu.2016.00227
Peptidylarginine Deiminase Inhibitor Suppresses Neutrophil Extracellular Trap Formation and MPO-ANCA Production
Abstract
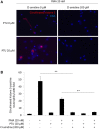
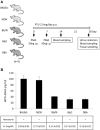
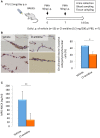
Myeloperoxidase-antineutrophil cytoplasmic antibody (MPO-ANCA)-associated vasculitis is a systemic small-vessel vasculitis, wherein, MPO-ANCA plays a critical role in the pathogenesis. Neutrophil extracellular traps (NETs) released from activated neutrophils are composed of extracellular web-like DNA and antimicrobial proteins, including MPO. Diverse stimuli, such as phorbol myristate acetate (PMA) and ligands of toll-like receptors (TLR), induce NETs. Although TLR-mediated NET formation can occur with preservation of living neutrophilic functions (called vital NETosis), PMA-stimulated neutrophils undergo cell death with NET formation (called suicidal NETosis). In the process of suicidal NETosis, histones are citrullinated by peptidylarginine deiminase 4 (PAD4). Since this step is necessary for decondensation of DNA, PAD4 plays a pivotal role in suicidal NETosis. Although NETs are essential for elimination of microorganisms, excessive formation of NETs has been suggested to be implicated in MPO-ANCA production. This study aimed to determine if pan-PAD inhibitors could suppress MPO-ANCA production in vivo. At first, NETs were induced in peripheral blood neutrophils derived from healthy donors (1 × 10(6)/ml) by stimulation with 20 nM PMA with or without 20 μM propylthiouracil (PTU), an anti-thyroid drug. We then determined that the in vitro NET formation was inhibited completely by 200 μM Cl-amidine, a pan-PAD inhibitor. Next, we established mouse models with MPO-ANCA production. BALB/c mice were given intraperitoneal (i.p.) injection of PMA (50 ng at days 0 and 7) and oral PTU (2.5 mg/day) for 2 weeks. These mice were divided into two groups; the first group was given daily i.p. injection of PBS (200 μl/day) (n = 13) and the other group with daily i.p. injection of Cl-amidine (0.3 mg/200 μl PBS/day) (n = 7). Two weeks later, citrullination as an indicator of NET formation in the peritoneum and serum MPO-ANCA titer was compared between the two groups. Results demonstrated that citrullination in the peritoneum was significantly reduced in the Cl-amidine-treated mice compared with the vehicle-injected control mice (38% reduction). Additionally, the serum MPO-ANCA titer of the Cl-amidine-treated mice (32.3 ± 31.0 ng/ml) was significantly lower than that in the vehicle-injected mice (132.1 ± 41.6 ng/ml). The collective findings indicate that excessive formation of NETs may be implicated in MPO-ANCA production in vivo.
Keywords: MPO-ANCA; MPO-ANCA-associated vasculitis; neutrophil extracellular trap; peptidylarginine deiminase 4; peptidylarginine deiminase inhibitor.
Figures
References
-
- Kallenberg CG. Pathogenesis and treatment of ANCA-associated vasculitides. Clin Exp Rheumatol (2015) 33:S11–4. - PubMed
-
- Nakazawa D, Tomaru U, Suzuki A, Masuda S, Hasegawa R, Kobayashi T, et al. Abnormal conformation and impaired degradation of propylthiouracil-induced neutrophil extracellular traps: implications of disordered neutrophil extracellular traps in a rat model of myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum (2012) 64:3779–87. 10.1002/art.34619 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

