Astigmatic multifocus microscopy enables deep 3D super-resolved imaging
- PMID: 27375935
- PMCID: PMC4918573
- DOI: 10.1364/BOE.7.002163
Astigmatic multifocus microscopy enables deep 3D super-resolved imaging
Abstract
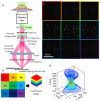
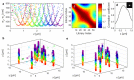
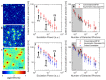
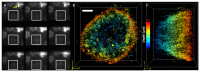
We have developed a 3D super-resolution microscopy method that enables deep imaging in cells. This technique relies on the effective combination of multifocus microscopy and astigmatic 3D single-molecule localization microscopy. We describe the optical system and the fabrication process of its key element, the multifocus grating. Then, two strategies for localizing emitters with our imaging method are presented and compared with a previously described deep 3D localization algorithm. Finally, we demonstrate the performance of the method by imaging the nuclear envelope of eukaryotic cells reaching a depth of field of ~4µm.
Keywords: (050.1380) Binary optics; (050.1950) Diffraction gratings; (100.6640) Superresolution; (110.1080) Active or adaptive optics; (180.2520) Fluorescence microscopy; (180.6900) Three-dimensional microscopy.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
