Dissociated functional significance of decision-related activity in the primate dorsal stream
- PMID: 27376476
- PMCID: PMC4966283
- DOI: 10.1038/nature18617
Dissociated functional significance of decision-related activity in the primate dorsal stream
Abstract
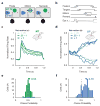
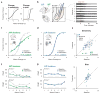
During decision making, neurons in multiple brain regions exhibit responses that are correlated with decisions. However, it remains uncertain whether or not various forms of decision-related activity are causally related to decision making. Here we address this question by recording and reversibly inactivating the lateral intraparietal (LIP) and middle temporal (MT) areas of rhesus macaques performing a motion direction discrimination task. Neurons in area LIP exhibited firing rate patterns that directly resembled the evidence accumulation process posited to govern decision making, with strong correlations between their response fluctuations and the animal's choices. Neurons in area MT, in contrast, exhibited weak correlations between their response fluctuations and choices, and had firing rate patterns consistent with their sensory role in motion encoding. The behavioural impact of pharmacological inactivation of each area was inversely related to their degree of decision-related activity: while inactivation of neurons in MT profoundly impaired psychophysical performance, inactivation in LIP had no measurable impact on decision-making performance, despite having silenced the very clusters that exhibited strong decision-related activity. Although LIP inactivation did not impair psychophysical behaviour, it did influence spatial selection and oculomotor metrics in a free-choice control task. The absence of an effect on perceptual decision making was stable over trials and sessions and was robust to changes in stimulus type and task geometry, arguing against several forms of compensation. Thus, decision-related signals in LIP do not appear to be critical for computing perceptual decisions, and may instead reflect secondary processes. Our findings highlight a dissociation between decision correlation and causation, showing that strong neuron-decision correlations do not necessarily offer direct access to the neural computations underlying decisions.
Figures







Comment in
-
Where Are Perceptual Decisions Made in the Brain?Trends Neurosci. 2016 Oct;39(10):642-644. doi: 10.1016/j.tins.2016.08.008. Epub 2016 Sep 9. Trends Neurosci. 2016. PMID: 27623195 Free PMC article. No abstract available.
-
Decision Activity in Parietal Cortex - Leader or Follower?Trends Cogn Sci. 2016 Nov;20(11):788-789. doi: 10.1016/j.tics.2016.09.005. Epub 2016 Sep 27. Trends Cogn Sci. 2016. PMID: 27686390
References
-
- Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci. 1996;13:87–100. - PubMed
-
- Shadlen MN, Newsome WT. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. Journal of Neurophysiology. 2001;86:1916–1936. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

