Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates
- PMID: 27377054
- PMCID: PMC5129521
- DOI: 10.1111/dom.12735
Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates
Abstract
Aims: To characterize the pharmacology of MEDI0382, a peptide dual agonist of glucagon-like peptide-1 (GLP-1) and glucagon receptors.
Materials and methods: MEDI0382 was evaluated in vitro for its ability to stimulate cAMP accumulation in cell lines expressing transfected recombinant or endogenous GLP-1 or glucagon receptors, to potentiate glucose-stimulated insulin secretion (GSIS) in pancreatic β-cell lines and stimulate hepatic glucose output (HGO) by primary hepatocytes. The ability of MEDI0382 to reduce body weight and improve energy balance (i.e. food intake and energy expenditure), as well as control blood glucose, was evaluated in mouse models of obesity and healthy cynomolgus monkeys following single and repeated daily subcutaneous administration for up to 2 months.
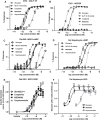
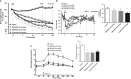
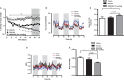
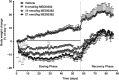
Results: MEDI0382 potently activated rodent, cynomolgus and human GLP-1 and glucagon receptors and exhibited a fivefold bias for activation of GLP-1 receptor versus the glucagon receptor. MEDI0382 produced superior weight loss and comparable glucose lowering to the GLP-1 peptide analogue liraglutide when administered daily at comparable doses in DIO mice. The additional fat mass reduction elicited by MEDI0382 probably results from a glucagon receptor-mediated increase in energy expenditure, whereas food intake suppression results from activation of the GLP-1 receptor. Notably, the significant weight loss elicited by MEDI0382 in DIO mice was recapitulated in cynomolgus monkeys.
Conclusions: Repeated administration of MEDI0382 elicits profound weight loss in DIO mice and non-human primates, produces robust glucose control and reduces hepatic fat content and fasting insulin and glucose levels. The balance of activities at the GLP-1 and glucagon receptors is considered to be optimal for achieving weight and glucose control in overweight or obese Type 2 diabetic patients.
Keywords: GLP-1 receptor knock-out mice; bodyweight; cynomolgus monkeys; diet-induced obese mice; dual agonist; glucagon; glucagon-like peptide-1; glucose tolerance; liraglutide; obesity; type 2 diabetes.
© 2016 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Figures


References
-
- Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819–837. - PubMed
-
- Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low‐calorie‐diet‐induced weight loss: the SCALE maintenance randomized study. Int J Obes. 2013;37:1443–1451. - PubMed
-
- Pi‐Sunyer X, Astrup A, Fujioka K, et al. SCALE obesity and prediabetes NN8022‐1839 study group. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11–22. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

