A spliceosome intermediate with loosely associated tri-snRNP accumulates in the absence of Prp28 ATPase activity
- PMID: 27377154
- PMCID: PMC4935976
- DOI: 10.1038/ncomms11997
A spliceosome intermediate with loosely associated tri-snRNP accumulates in the absence of Prp28 ATPase activity
Abstract
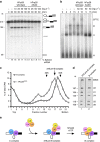
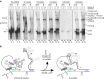
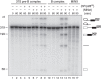
The precise role of the spliceosomal DEAD-box protein Prp28 in higher eukaryotes remains unclear. We show that stable tri-snRNP association during pre-catalytic spliceosomal B complex formation is blocked by a dominant-negative hPrp28 mutant lacking ATPase activity. Complexes formed in the presence of ATPase-deficient hPrp28 represent a novel assembly intermediate, the pre-B complex, that contains U1, U2 and loosely associated tri-snRNP and is stalled before disruption of the U1/5'ss base pairing interaction, consistent with a role for hPrp28 in the latter. Pre-B and B complexes differ structurally, indicating that stable tri-snRNP integration is accompanied by substantial rearrangements in the spliceosome. Disruption of the U1/5'ss interaction alone is not sufficient to bypass the block by ATPase-deficient hPrp28, suggesting hPrp28 has an additional function at this stage of splicing. Our data provide new insights into the function of Prp28 in higher eukaryotes, and the requirements for stable tri-snRNP binding during B complex formation.
Figures




References
-
- Wahl M. C., Will C. L. & Lührmann R. The spliceosome: design principles of a dynamic RNP machine. Cell 136, 701–718 (2009). - PubMed
-
- Nilsen T. W. RNA Structure and Function 279–307Cold Spring Harbor Laboratory Press (1998).
-
- Valadkhan S. snRNAs as the catalysts of pre-mRNA splicing. Curr. Opin. Chem. Biol. 9, 603–608 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

