The zinc sensing receptor, ZnR/GPR39, triggers metabotropic calcium signalling in colonocytes and regulates occludin recovery in experimental colitis
- PMID: 27377730
- PMCID: PMC4938021
- DOI: 10.1098/rstb.2015.0420
The zinc sensing receptor, ZnR/GPR39, triggers metabotropic calcium signalling in colonocytes and regulates occludin recovery in experimental colitis
Erratum in
-
Correction to 'The zinc sensing receptor, ZnR/GPR39, triggers metabotropic calcium signalling in colonocytes and regulates occludin recovery in experimental colitis'.Philos Trans R Soc Lond B Biol Sci. 2016 Sep 19;371(1703):20160331. doi: 10.1098/rstb.2016.0331. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27502386 Free PMC article. No abstract available.
Abstract
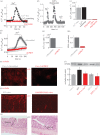
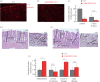
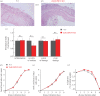
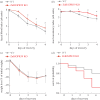
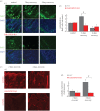
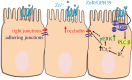
Impaired epithelial barrier function is a hallmark of inflammatory bowel diseases, such as colitis, contributing to diarrhoea and perpetuating inflammation. We show that the zinc sensing receptor, ZnR/GPR39, triggers intracellular Ca(2+) signalling in colonocytes thereby inducing occludin expression. Moreover, ZnR/GPR39 is essential for epithelial barrier recovery in the dextran sodium sulfate (DSS) ulcerative colitis model. Loss of ZnR/GPR39 results in increased susceptibility to DSS-induced inflammation, owing to low expression of the tight junction protein occludin and impaired epithelial barrier. Recovery of wild-type (WT) mice from the DSS insult was faster than that of ZnR/GPR39 knockout (KO) mice. Enhanced recovery of the epithelial layer and increased crypt regeneration were observed in WT mice compared with ZnR/GPR39 KO, suggesting that ZnR/GPR39 is promoting epithelial barrier integrity following DSS insult. Indeed, cell proliferation and apical expression of occludin, following the DSS-induced epithelial erosion, were increased in WT tissue but not in ZnR/GPR39 KO tissue. Importantly, survival following DSS treatment was higher in WT mice compared with ZnR/GPR39 KO mice. Our results support a direct role for ZnR/GPR39 in promoting epithelial renewal and barrier function following DSS treatment, thereby affecting the severity of the disease. We suggest ZnR/GPR39 as a novel therapeutic target that can improve epithelial barrier function in colitis.This article is part of the themed issue 'Evolution brings Ca(2+) and ATP together to control life and death'.
Keywords: Ca2+ signalling; ZnR/GPR39; colitis; intestinal epithelium; tight junction; zinc.
© 2016 The Author(s).
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

