In vivo label-free measurement of lymph flow velocity and volumetric flow rates using Doppler optical coherence tomography
- PMID: 27377852
- PMCID: PMC4932526
- DOI: 10.1038/srep29035
In vivo label-free measurement of lymph flow velocity and volumetric flow rates using Doppler optical coherence tomography
Abstract
Direct in vivo imaging of lymph flow is key to understanding lymphatic system function in normal and disease states. Optical microscopy techniques provide the resolution required for these measurements, but existing optical techniques for measuring lymph flow require complex protocols and provide limited temporal resolution. Here, we describe a Doppler optical coherence tomography platform that allows direct, label-free quantification of lymph velocity and volumetric flow rates. We overcome the challenge of very low scattering by employing a Doppler algorithm that operates on low signal-to-noise measurements. We show that this technique can measure lymph velocity at sufficiently high temporal resolution to resolve the dynamic pulsatile flow in collecting lymphatic vessels.
Figures

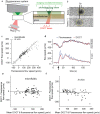
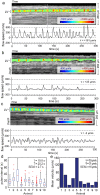
 and S.E.M. denote mean, median and standard error of the mean respectively.
and S.E.M. denote mean, median and standard error of the mean respectively.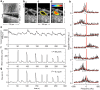
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

