Anti-Osteoporosis Medication Prescriptions and Incidence of Subsequent Fracture Among Primary Hip Fracture Patients in England and Wales: An Interrupted Time-Series Analysis
- PMID: 27377877
- PMCID: PMC5122450
- DOI: 10.1002/jbmr.2882
Anti-Osteoporosis Medication Prescriptions and Incidence of Subsequent Fracture Among Primary Hip Fracture Patients in England and Wales: An Interrupted Time-Series Analysis
Abstract
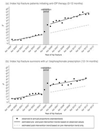
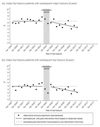
In January 2005, the National Institute for Health and Care Excellence (NICE) in England and Wales provided new guidance on the use of antiosteoporosis therapies for the secondary prevention of osteoporotic fractures. This was shortly followed in the same year by market authorization of a generic form of alendronic acid within the UK. We here set out to estimate the actual practice impact of these events among hip fracture patients in terms of antiosteoporosis medication prescribing and subsequent fracture incidence using primary care data (Clinical Practice Research Datalink) from 1999 to 2013. Changes in level and trend of prescribing and subsequent fracture following publication of NICE guidance and availability of generic alendronic acid were estimated using an interrupted time series analysis. Both events were considered in combination within a 1-year "intervention period." We identified 10,873 primary hip fracture patients between April 1999 and Sept 2012. Taking into account prior trend, the intervention period was associated with an immediate absolute increase of 14.9% (95% CI, 10.9 to 18.9) for incident antiosteoporosis prescriptions and a significant and clinically important reduction in subsequent major and subsequent hip fracture: -0.19% (95% CI, -0.28 to -0.09) and -0.17% (95% CI, -0.26 to -0.09) per 6 months, respectively. This equated to an approximate 14% (major) and 22% (hip) reduction at 3 years postintervention relative to expected values based solely on preintervention level and trend. We conclude that among hip fracture patients, publication of NICE guidance and availability of generic alendronic acid was temporally associated with increased prescribing and a significant decline in subsequent fractures. © 2016 American Society for Bone and Mineral Research.
Keywords: BISPHOSPHONATES; EPIDEMIOLOGY; GUIDELINES; HEALTH SERVICES RESEARCH; HIP FRACTURE; OSTEOPOROSIS; SECONDARY PREVENTION.
© 2016 American Society for Bone and Mineral Research.
Conflict of interest statement
Declaration of interests JL, DPA, NKA, CC, MKJ and AJ received grants from NIHR HS&DR during the conduct of the study. Outside the submitted work, MKJ reports personal fees from Lilly UK, Amgen, Sevier, Merck, Medtronic, Internis, Consilient Health and serves on the Scientific Committee of the National Osteoporosis Society and International Osteoporosis Foundation; DPA received grants from Bioiberica S.A. and Amgen Spain S.A.; CC received personal fees from Servier, Amgen, Eli Lilly, Merck, Medtronic and Novartis. NKA reports personal fees from Merck, Smith and Nephew, Q-Med, Nicox, Flexion, Bioiberica, Servier and grants and personal fees from Roche. AJ has received consultancy, lecture fees and honoraria from Servier, UK Renal Registry, Oxford Craniofacial Unit, IDIAP Jordi Gol, Freshfields Bruckhaus Deringer, has held advisory board positions (which involved receipt of fees) from Anthera Pharmaceuticals, INC., and received research sponsorship from ROCHE. SH and AD have no competing financial interests relevant to the submitted work.
Figures

References
-
- The care of patients with fragility fractures: British Orthopaedic Association (BOA) 2007 [Available from: http://www.bgs.org.uk/pdf_cms/pubs/Blue Book on fragility fracture care.pdf.
-
- Leal J, Gray AM, Prieto-Alhambra D, Arden NK, Cooper C, Javaid MK, et al. Impact of hip fracture on hospital care costs: a population-based study. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2016;27(2):549–58. - PMC - PubMed
-
- Griffin XL, Parsons N, Achten J, Fernandez M, Costa ML. Recovery of health-related quality of life in a United Kingdom hip fracture population. The Warwick Hip Trauma Evaluation--a prospective cohort study. Bone Joint J. 2015;97-B(3):372–82. - PubMed
-
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. Journal of Bone and Mineral Research. 2007;22(3):465–75. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

