A clinical tool for predicting survival in ALS
- PMID: 27378085
- PMCID: PMC5136716
- DOI: 10.1136/jnnp-2015-312908
A clinical tool for predicting survival in ALS
Abstract
Background: Amyotrophic lateral sclerosis (ALS) is a progressive and usually fatal neurodegenerative disease. Survival from diagnosis varies considerably. Several prognostic factors are known, including site of onset (bulbar or limb), age at symptom onset, delay from onset to diagnosis and the use of riluzole and non-invasive ventilation (NIV). Clinicians and patients would benefit from a practical way of using these factors to provide an individualised prognosis.
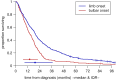
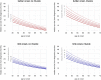
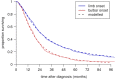
Methods: 575 consecutive patients with incident ALS from a population-based registry in South-East England register for ALS (SEALS) were studied. Their survival was modelled as a two-step process: the time from diagnosis to respiratory muscle involvement, followed by the time from respiratory involvement to death. The effects of predictor variables were assessed separately for each time interval.
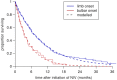
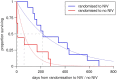
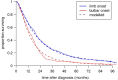
Findings: Younger age at symptom onset, longer delay from onset to diagnosis and riluzole use were associated with slower progression to respiratory involvement, and NIV use was associated with lower mortality after respiratory involvement, each with a clinically significant effect size. Riluzole may have a greater effect in younger patients and those with longer delay to diagnosis. A patient's survival time has a roughly 50% chance of falling between half and twice the predicted median.
Interpretation: A simple and clinically applicable graphical method of predicting an individual patient's survival from diagnosis is presented. The model should be validated in an independent cohort, and extended to include other important prognostic factors.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
PNL reports grants from MND Association Funding to support MND Clinic from 1995 to 2010, during the conduct of the study. CES reports consultancy and/or collaborative relationships with Glaxo Smith Kline, Vertex Pharmaceuticals, Chronos Therapeutics and Eli Lilly; he receives no personal gain and has never had any shares of any sort. AAC reports grants from EU FP7, grants from EU JPND—MRC and ESRC, grants from NIHR BRC in Mental Health and Dementia BRU, during the conduct of the study. All other authors declare no conflicts of interest.
Figures
Comment in
-
Clinical tool for predicting survival in ALS: do we need one?J Neurol Neurosurg Psychiatry. 2016 Dec;87(12):1275. doi: 10.1136/jnnp-2016-313683. Epub 2016 Jul 4. J Neurol Neurosurg Psychiatry. 2016. PMID: 27378084 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
