Transcutaneous electrical stimulation (TES) for treatment of constipation in children
- PMID: 27378432
- PMCID: PMC6457877
- DOI: 10.1002/14651858.CD010873.pub2
Transcutaneous electrical stimulation (TES) for treatment of constipation in children
Abstract
Background: Childhood constipation is a common problem with substantial health, economic and emotional burdens. Existing therapeutic options, mainly pharmacological, are not consistently effective, and some are associated with adverse effects after prolonged use. Transcutaneous electrical stimulation (TES), a non-pharmacological approach, is postulated to facilitate bowel movement by modulating the nerves of the large bowel via the application of electrical current transmitted through the abdominal wall.
Objectives: Our main objective was to evaluate the effectiveness and safety of TES when employed to improve bowel function and constipation-related symptoms in children with constipation.
Search methods: We searched MEDLINE (PubMed) (1950 to July 2015), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, Issue 7, 2015), EMBASE (1980 to July 2015), the Cochrane IBD Group Specialized Register, trial registries and conference proceedings to identify applicable studies .
Selection criteria: Randomized controlled trials that assessed any type of TES, administered at home or in a clinical setting, compared to no treatment, a sham TES, other forms of nerve stimulation or any other pharmaceutical or non-pharmaceutical measures used to treat constipation in children were considered for inclusion.
Data collection and analysis: Two authors independently assessed studies for inclusion, extracted data and assessed risk of bias of the included studies. We calculated the risk ratio (RR) and corresponding 95% confidence interval (CI) for categorical outcomes data and the mean difference (MD) and corresponding 95% CI for continuous outcomes.
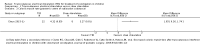
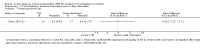
Main results: One study from Australia including 46 children aged 8 to 18 years was eligible for inclusion. There were multiple reports identified, including one unpublished report, that focused on different outcomes of the same study. The study had unclear risk of selection bias, high risks of performance, detection and attrition biases, and low risks of reporting biases.There were no significant differences between TES and the sham control group for the following outcomes: i).number of children with > 3 complete spontaneous bowel movements (CSBM) per week (RR 1.07, 95% CI 0.74 to 1.53, one study, 42 participants) (
Quality of evidence: very low, due to high risk of bias and serious imprecision ), ii). number of children with improved colonic transit assessed radiologically (RR 5.00, 95% CI 0.79 to 31.63; one study, 21 participants) (
Quality of evidence: very low, due to high risk of bias, serious imprecision and indirectness of the outcome). However, mean colonic transit rate, measured as the position of the geometric centre of the radioactive substance ingested along the intestinal tract, was significantly higher in children who received TES compared to sham (MD 1.05, 95% CI 0.36 to 1.74; one study, 30 participants) (
Quality of evidence: very low, due to high risk of bias , serious imprecision and indirectness of the outcome). There was no significant difference between the two groups in the number of children with improved soiling-related symptoms (RR 2.08, 95% CI 0.86 to 5.00; one study, 25 participants) (
Quality of evidence: very low, due to high risk of bias and serious imprecision). There was no significant difference in the number of children with improved quality of life (QoL) (RR 4.00, 95% CI 0.56 to 28.40; one study, 16 participants) (
Quality of evidence: very low, due to high risk of bias issues and serious imprecision ). There were also no significant differences in in self-perceived (MD 5.00, 95% CI -1.21 to 11.21) or parent-perceived QoL (MD -0.20, 95% CI -7.57 to 7.17, one study, 33 participants for both outcomes) (QUALITY OF EVIDENCE for both outcomes: very low, due to high risk of bias and serious imprecision). No adverse effects were reported in the included study.
Authors' conclusions: The very low quality evidence gathered in this review does not suggest that TES provides a benefit for children with chronic constipation. Further randomized controlled trials assessing TES for the management of childhood constipation should be conducted. Future trials should include clear documentation of methodologies, especially measures to evaluate the effectiveness of blinding, and incorporate patient-important outcomes such as the number of patients with improved CSBM, improved clinical symptoms and quality of life.
Conflict of interest statement
Ruey Terng Ng: None known,
Way Seah Lee: None known
Hak Lee Ang: None known
Kai Ming Teo: has stock or stock options held by investment firms whose investment might or might not include pharmaceutical companies or relevant companies; has received travel expenses from milk companies to to attend paediatric or gastroenterology conferences; and has received funds from milk and drug companies to attend lunch and dinner meetings and conferences. These financial activities are outside the scope of this review.
Yee Ian Yik has an intellectual conflict of interest, being an author of the included study. However, he was not involved in determination of eligibility, risk of bias assessment or data extraction.
Nai Ming Lai: None known
Figures







Update of
- doi: 10.1002/14651858.CD010873
References
References to studies included in this review
-
- Chase J, Gibb S M, Clarke M C C, Catto‐Smith A, Robertson V, Hutson J M, et al. Transcutaneous electrical current to overcome slow transit constipation in children ‐ An RCT. Neurogastroenterology and Motility 2009;21:78. - PubMed
- Chase JW, Clarke MCC, Gibb S, Hutson JM, Catto‐Smith AG, Robertson V, et al. Transcutaneous electrical stimulation to treat childhood slow transit constipation ‐ a randomised placebo controlled trial (Abstract). Australian and New Zealand Continence Journal. 2008:114.
- Chase JW, Clarke MCC, Gibb SM, Catto‐Smith AG, Robertson VJ, King SK, et al. Transabdominal electrical stimulation increases colonic activity in paediatric slow transit constipation. Data on file. - PubMed
- Clarke M C, Chase J W, Gibb S, Robertson V J, Catto‐Smith A, Hutson J M, et al. Decreased colonic transit time after transcutaneous interferential electrical stimulation in children with slow transit constipation. Journal of Pediatric Surgery 2009;44:408‐12. - PubMed
- Clarke MC, Chase JW, Gibb S, Hutson JM, Southwell BR. Improvement of quality of life in children with slow transit constipation after treatment with transcutaneous electrical stimulation. Journal of Pediatric Surgery 2009;44:1268‐72; discussion 1272. - PubMed
- Leong L C, Yik Y I, Catto‐Smith A G, Robertson V J, Hutson J M, Southwell B R. Long‐term effects of transabdominal electrical stimulation in treating children with slow‐transit constipation. Journal of Pediatric Surgery 2011;46:2309‐12. - PubMed
- Leong LCY, Yik YI, Farmer P, Hutson JM, Southwell BR. Long‐term effects of transabdominal electrical stimulation on paediatric slow‐transit constipation. Journal of Gastroenterology and Hepatology 2011;26:91.
- Southwell B. Transabdominal electrical stimulation to treat slow transit constipation in children. Neuromodulation 2012;15:63.
- Southwell B R. Transabdominal electrical stimulation increases colonic activity in pediatric slow transit constipation: Results from a blinded randomised‐control trial. Gastroenterology 2010;138:S109.
- Southwell BR, Clarke MC, Chase J, Hutson JM. Transcutaneous electrical current to overcome slow transit constipation in children. Neurogastroenterology and Motility. Blackwell Publishing Ltd, 2008:735ii. [DOI: 10.1111/j.1365-2982.2008.01121.x] - DOI
- Yik YI, Clarke MC, Catto‐Smith AG, Robertson VJ, Sutcliffe JR, Chase JW, et al. Slow‐transit constipation with concurrent upper gastrointestinal dysmotility and its response to transcutaneous electrical stimulation. Pediatric Surgery International 2011;27:705‐11. - PubMed
References to studies excluded from this review
-
- Chase J, Robertson V J, Southwell B, Hutson J, Gibb S. Pilot study using transcutaneous electrical stimulation (interferential current) to treat chronic treatment‐resistant constipation and soiling in children. Journal of Gastroenterology and Hepatology 2005;20:1054‐61. - PubMed
-
- Chase J, Gibb S, Ismail KA, Clarke MCC, Catto‐Smith AG, Robertson VJ, et al. Daily transcutaneous electrical stimulation (using interferential current) at home increased defecation in children with slow transit constipation: A pilot study. Neurogastroenterology and Motility 2009;21:3.
-
- Clarke MC, Catto‐Smith AG, King SK, Dinning PG, Cook IJ, Chase JW, et al. Transabdominal electrical stimulation increases colonic propagating pressure waves in paediatric slow transit constipation. Journal of Pediatric Surgery 2012;47:2279‐84. - PubMed
-
- Dwyer ME, Vandersteen DR, Hollatz P, Reinberg YE. Sacral neuromodulation for the dysfunctional elimination syndrome: a 10‐year single‐center experience with 105 consecutive children. Urology 2014;84:911‐7. - PubMed
-
- Ismail KA, Chase J, Gibb S, Clarke M, Catto‐Smith AG, Robertson VJ, et al. Daily transabdominal electrical stimulation at home increased defecation in children with slow‐transit constipation: a pilot study. Journal of Pediatric Surgery 2009;44:2388‐92. - PubMed
Additional references
-
- Eleouet M, Siproudhis L, Guillou N, Couedic J, Bouguen G, Bretagne JF. Chronic posterior tibial nerve transcutaneous electrical nerve stimulation (TENS) to treat fecal incontinence (FI). International Journal of Colorectal Disease 2010;25(9):1127‐32. [PUBMED: 20549220] - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

