Ectodysplasin signalling deficiency in mouse models of hypohidrotic ectodermal dysplasia leads to middle ear and nasal pathology
- PMID: 27378689
- PMCID: PMC5179950
- DOI: 10.1093/hmg/ddw202
Ectodysplasin signalling deficiency in mouse models of hypohidrotic ectodermal dysplasia leads to middle ear and nasal pathology
Abstract
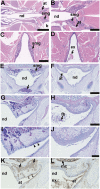
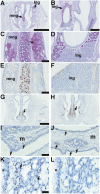
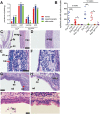
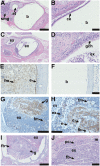
Hypohidrotic ectodermal dysplasia (HED) results from mutation of the EDA, EDAR or EDARADD genes and is characterized by reduced or absent eccrine sweat glands, hair follicles and teeth, and defective formation of salivary, mammary and craniofacial glands. Mouse models with HED also carry Eda, Edar or Edaradd mutations and have defects that map to the same structures. Patients with HED have ear, nose and throat disease, but this has not been investigated in mice bearing comparable genetic mutations. We report that otitis media, rhinitis and nasopharyngitis occur at high frequency in Eda and Edar mutant mice and explore the pathogenic mechanisms related to glandular function, microbial and immune parameters in these lines. Nasopharynx auditory tube glands fail to develop in HED mutant mice and the functional implications include loss of lysozyme secretion, reduced mucociliary clearance and overgrowth of nasal commensal bacteria accompanied by neutrophil exudation. Heavy nasopharynx foreign body load and loss of gland protection alters the auditory tube gating function and the auditory tubes can become pathologically dilated. Accumulation of large foreign body particles in the bulla stimulates granuloma formation. Analysis of immune cell populations and myeloid cell function shows no evidence of overt immune deficiency in HED mutant mice. Our findings using HED mutant mice as a model for the human condition support the idea that ear and nose pathology in HED patients arises as a result of nasal and nasopharyngeal gland deficits, reduced mucociliary clearance and impaired auditory tube gating function underlies the pathological sequelae in the bulla.
© The Author 2016. Published by Oxford University Press.
Figures



References
-
- Sadier A., Viriot L., Pantalacci S., Laudet V. (2014) The ectodysplasin pathway: from diseases to adaptations. Trends Genet, 30, 24–31. - PubMed
-
- Majumder K., Shawlot W., Schuster G., Harrison W., Elder F.F., Overbeek P.A. (1998) YAC rescue of downless locus mutations in mice. Mamm. Genome., 9, 863–868. - PubMed
-
- Mou C., Thomason H.A., Willan P.M., Clowes C., Harris W.E., Drew C.F., Dixon J., Dixon M.J., Headon D.J. (2008) Enhanced ectodysplasin-A receptor (EDAR) signaling alters multiple fiber characteristics to produce the East Asian hair form. Hum. Mutat., 29, 1405–1411. - PubMed
-
- Mehta U., Brunworth J., Lewis R.A., Sindwani R. (2007) Rhinologic manifestations of ectodermal dysplasia. Am. J. Rhinol., 21, 55–58. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

