Dystrophin contains multiple independent membrane-binding domains
- PMID: 27378693
- PMCID: PMC5216612
- DOI: 10.1093/hmg/ddw210
Dystrophin contains multiple independent membrane-binding domains
Abstract
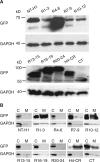
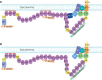
Dystrophin is a large sub-sarcolemmal protein. Its absence leads to Duchenne muscular dystrophy (DMD). Binding to the sarcolemma is essential for dystrophin to protect muscle from contraction-induced injury. It has long been thought that membrane binding of dystrophin depends on its cysteine-rich (CR) domain. Here, we provide in vivo evidence suggesting that dystrophin contains three additional membrane-binding domains including spectrin-like repeats (R)1-3, R10-12 and C-terminus (CT). To systematically study dystrophin membrane binding, we split full-length dystrophin into ten fragments and examined subcellular localizations of each fragment by adeno-associated virus-mediated gene transfer. In skeletal muscle, R1-3, CR domain and CT were exclusively localized at the sarcolemma. R10-12 showed both cytosolic and sarcolemmal localization. Importantly, the CR-independent membrane binding was conserved in murine and canine muscles. A critical function of the CR-mediated membrane interaction is the assembly of the dystrophin-associated glycoprotein complex (DGC). While R1-3 and R10-12 did not restore the DGC, surprisingly, CT alone was sufficient to establish the DGC at the sarcolemma. Additional studies suggest that R1-3 and CT also bind to the sarcolemma in the heart, though relatively weak. Taken together, our study provides the first conclusive in vivo evidence that dystrophin contains multiple independent membrane-binding domains. These structurally and functionally distinctive membrane-binding domains provide a molecular framework for dystrophin to function as a shock absorber and signaling hub. Our results not only shed critical light on dystrophin biology and DMD pathogenesis, but also provide a foundation for rationally engineering minimized dystrophins for DMD gene therapy.
© The Author 2016. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures




References
-
- Hoffman E.P., Brown R.H.J., Kunkel L.M. (1987) Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell, 51, 919–928. - PubMed
-
- Koenig M., Kunkel L.M. (1990) Detailed analysis of the repeat domain of dystrophin reveals four potential hinge segments that may confer flexibility. J. Biol. Chem., 265, 4560–4566. - PubMed
-
- Amann K.J., Renley B.A., Ervasti J.M. (1998) A cluster of basic repeats in the dystrophin rod domain binds F-actin through an electrostatic interaction. J Biol. Chem., 273, 28419–28423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

