Pharmacokinetic and Pharmacodynamic Properties of a Novel Inhaled Insulin
- PMID: 27378794
- PMCID: PMC5375067
- DOI: 10.1177/1932296816658055
Pharmacokinetic and Pharmacodynamic Properties of a Novel Inhaled Insulin
Abstract
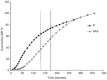
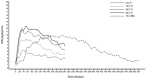
Advances in insulin treatment options over recent decades have markedly improved the management of diabetes. Despite this, glycemic control remains suboptimal in many people with diabetes. Although postprandial glucose control has been improved with the development of subcutaneously injected rapid-acting insulin analogs, currently available insulins are not able to fully mimic the physiological time-action profile of endogenously secreted insulin after a meal. The delayed onset of metabolic action and prolonged period of effect induce the risk of postprandial hyperglycemia and late postprandial hypoglycemia. A number of alternative routes of insulin administration have been investigated over time in an attempt to overcome the limitations associated with subcutaneous administration and to provide an improved time-action insulin profile more closely simulating physiological prandial insulin release. Among these, pulmonary insulin delivery has shown the most promise. Technosphere® Inhaled Insulin (TI) is a rapid-acting inhaled human insulin recently approved by the FDA for prandial insulin therapy. In this article we discuss the pharmacokinetic and pharmacodynamic properties of TI, and, based on key studies performed during its clinical development, the implications for improved postprandial glucose control.
Keywords: inhaled insulins; insulin therapy; pharmacodynamics; pharmacokinetics; prandial insulin.
Conflict of interest statement
Figures



References
-
- Heise T, Pieber TR. Towards peakless, reproducible and long-acting insulins. An assessment of the basal analogues based on isoglycaemic clamp studies. Diabetes Obes Metab. 2007;9:648-659. - PubMed
-
- Owens DR, Matfin G, Monnier L. Basal insulin analogues in the management of diabetes mellitus: What progress have we made? Diabetes Metab Res Rev. 2014;30:104-119. - PubMed
-
- Pettus J, Santos Cavaiola T, Tamborlane WV, Edelman S. The past, present, and future of basal insulins. Diabetes Metab Res Rev. 2016;32:478-96. - PubMed
-
- Heinemann L, Heise T, Wahl CH, et al. Prandial glycaemia after a carbohydrate-rich meal in type I diabetic patients using the rapid acting insulin analogue [Lys(B28), Pro(B29)] human insulin. Diabet Med. 1996;3:625-629. - PubMed
-
- Chen JW, Christiansen JS, Lauritzen T. Limitations to subcutaneous insulin administration in type 1 diabetes. Diabetes Obes Metab. 2003;5:223-233. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

