The Revolution in Migraine Genetics: From Aching Channels Disorders to a Next-Generation Medicine
- PMID: 27378853
- PMCID: PMC4904011
- DOI: 10.3389/fncel.2016.00156
The Revolution in Migraine Genetics: From Aching Channels Disorders to a Next-Generation Medicine
Abstract
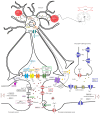
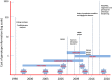
Channelopathies are a heterogeneous group of neurological disorders resulting from dysfunction of ion channels located in cell membranes and organelles. The clinical scenario is broad and symptoms such as generalized epilepsy (with or without fever), migraine (with or without aura), episodic ataxia and periodic muscle paralysis are some of the best known consequences of gain- or loss-of-function mutations in ion channels. We review the main clinical effects of ion channel mutations associated with a significant impact on migraine headache. Given the increasing and evolving use of genetic analysis in migraine research-greater emphasis is now placed on genetic markers of dysfunctional biological systems-we also show how novel information in rare monogenic forms of migraine might help to clarify the disease mechanisms in the general population of migraineurs. Next-generation sequencing (NGS) and more accurate and precise phenotyping strategies are expected to further increase understanding of migraine pathophysiology and genetics.
Keywords: astrocyte; calcium channel; glutamate; migraine; sodium channel; tripartite synapse.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

