Validation of a Stability-Indicating RP-HPLC Method for Determination of l-Carnitine in Tablets
- PMID: 27379281
- PMCID: PMC4897466
- DOI: 10.1155/2014/481059
Validation of a Stability-Indicating RP-HPLC Method for Determination of l-Carnitine in Tablets
Abstract
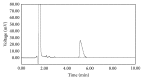
A rapid and stability-indicating RP-HPLC method was developed for determination of l-carnitine in tablets. The separation was based on a C18 analytical column using a mobile phase which consisted of 0.05 M phosphate buffer (pH = 3): ethanol (99 : 1), including 0.56 mg/mL of sodium 1-heptanesulfonate. Column temperature was set at 50°C and quantitation was achieved by UV detection at 225 nm. In forced degradation studies, the drug was subjected to oxidation, hydrolysis, photolysis, and heat. Among the different stress conditions, the exposure to acidic and basic conditions was found to be an important adverse stability factor. The method was validated for specificity, selectivity, linearity, precision, accuracy, and robustness. The applied procedure was found to be linear in l-carnitine concentration range of 84.74-3389.50 µg/mL (r (2) = 0.9997). Precision was evaluated by replicate analysis in which relative standard deviation (RSD) values for areas were found below 2.0%. The recoveries obtained (100.83%-101.54%) ensured the accuracy of the developed method. The expanded uncertainty (3.14%) of the method was also estimated from method validation data. Accordingly, the proposed validated and rapid procedure was proved to be suitable for routine analyzing and stability studies of l-carnitine in tablets.
Figures




References
-
- de Andrés F., Castañeda G., Ríos G. A. Achiral liquid chromatography with circular dichroism detection for the determination of carnitine enantiomers in dietary supplements and pharmaceutical formulations. Journal of Pharmaceutical and Biomedical Analysis. 2010;51(2):478–483. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

