Single-Electron Transmetalation via Photoredox/Nickel Dual Catalysis: Unlocking a New Paradigm for sp(3)-sp(2) Cross-Coupling
- PMID: 27379472
- PMCID: PMC4955519
- DOI: 10.1021/acs.accounts.6b00214
Single-Electron Transmetalation via Photoredox/Nickel Dual Catalysis: Unlocking a New Paradigm for sp(3)-sp(2) Cross-Coupling
Abstract
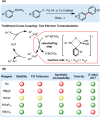
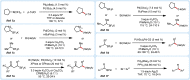
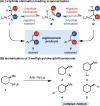
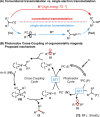
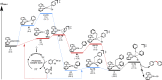
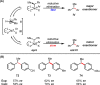
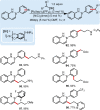
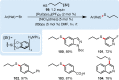
The important role of transition metal-catalyzed cross-coupling in expanding the frontiers of accessible chemical territory is unquestionable. Despite empowering chemists with Herculean capabilities in complex molecule construction, contemporary protocols are not without their Achilles' heel: Csp(3)-Csp(2)/sp(3) coupling. The underlying challenge in sp(3) cross-couplings is 2-fold: (i) methods employing conventional, bench-stable precursors are universally reliant on extreme reaction conditions because of the high activation barrier of transmetalation; (ii) circumvention of this barrier invariably relies on use of more reactive precursors, thereby sacrificing functional group tolerance, operational simplicity, and broad applicability. Despite the ubiquity of this problem, the nature of the transmetalation step has remained unchanged from the seminal reports of Negishi, Suzuki, Kumada, and Stille, thus suggesting that the challenges in Csp(3)-Csp(2)/sp(3) coupling result from inherent mechanistic constraints in the traditional cross-coupling paradigm. Rather than submitting to the limitations of this conventional approach, we envisioned that a process rooted in single-electron reactivity could furnish the same key metalated intermediate posited in two-electron transmetalation, while demonstrating entirely complementary reactivity patterns. Inspired by literature reports on the susceptibility of organoboron reagents toward photochemical, single-electron oxidative fragmentation, realization of a conceptually novel open shell transmetalation framework was achieved in the facile coupling of benzylic trifluoroborates with aryl halides via cooperative visible-light activated photoredox and Ni cross-coupling catalysis. Following this seminal study, we disclosed a suite of protocols for the cross-coupling of secondary alkyl, α-alkoxy, α-amino, and α-trifluoromethylbenzyltrifluoroborates. Furthermore, the selective cross-coupling of Csp(3) organoboron moieties in the presence of Csp(2) organoboron motifs was also demonstrated, highlighting the nuances of this approach to transmetalation. Computational modeling of the reaction mechanism uncovered useful details about the intermediates and transition-state structures involved in the nickel catalytic cycle. Most notably, a unique dynamic kinetic resolution process, characterized by radical homolysis/recombination equilibrium of a Ni(III) intermediate, was discovered. This process was ultimately found to be responsible for stereoselectivity in an enantioselective variant of these cross-couplings. Prompted by the intrinsic limitations of organotrifluoroborates, we sought other radical feedstocks and quickly identified alkylbis(catecholato)silicates as viable radical precursors for Ni/photoredox dual catalysis. These hypervalent silicate species have several notable benefits, including more favorable redox potentials that allow extension to primary alkyl systems incorporating unprotected amines as well as compatibility with less expensive Ru-based photocatalysts. Additionally, these reagents exhibit an amenability to alkenyl halide cross-coupling while simultaneously expanding the aryl halide scope. In the process of exploring these reagents, we serendipitously discovered a method to effect thioetherification of aryl halides via a H atom transfer mechanism. This latter discovery emphasizes that this robust cross-coupling paradigm is "blind" to the origins of the radical, opening opportunities for a wealth of new discoveries. Taken together, our studies in the area of photoredox/nickel dual catalysis have validated single-electron transmetalation as a powerful platform for enabling conventionally challenging Csp(3)-Csp(2) cross-couplings. More broadly, these findings represent the power of rational design in catalysis and the strategic use of mechanistic knowledge and manipulation for the development of new synthetic methods.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
-
For reviews on this topic, see:
- Maluenda I.; Navarro O. Recent Developments in the Suzuki-Miyaura Reaction: 2010–2014. Molecules 2015, 20, 7528–7557. 10.3390/molecules20057528. - DOI - PMC - PubMed
- Miyaura N.; Suzuki A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. 10.1021/cr00039a007. - DOI
-
-
- Tamao K.; Sumitani K.; Kiso Y.; Zembayashi M.; Fujioka A.; Kodama S.; Nakajima I.; Minato A.; Kumada M. Nickel-Phosphine Complex-Catalyzed Grignard Coupling. I. Cross-Coupling of Alkyl, Aryl, and Alkenyl Grignard Reagents with Aryl and Alkenyl Halides: General Scope and Limitations. Bull. Chem. Soc. Jpn. 1976, 49, 1958–1969. 10.1246/bcsj.49.1958. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

