Global identification of hnRNP A1 binding sites for SSO-based splicing modulation
- PMID: 27380775
- PMCID: PMC4932749
- DOI: 10.1186/s12915-016-0279-9
Global identification of hnRNP A1 binding sites for SSO-based splicing modulation
Abstract
Background: Many pathogenic genetic variants have been shown to disrupt mRNA splicing. Besides splice mutations in the well-conserved splice sites, mutations in splicing regulatory elements (SREs) may deregulate splicing and cause disease. A promising therapeutic approach is to compensate for this deregulation by blocking other SREs with splice-switching oligonucleotides (SSOs). However, the location and sequence of most SREs are not well known.
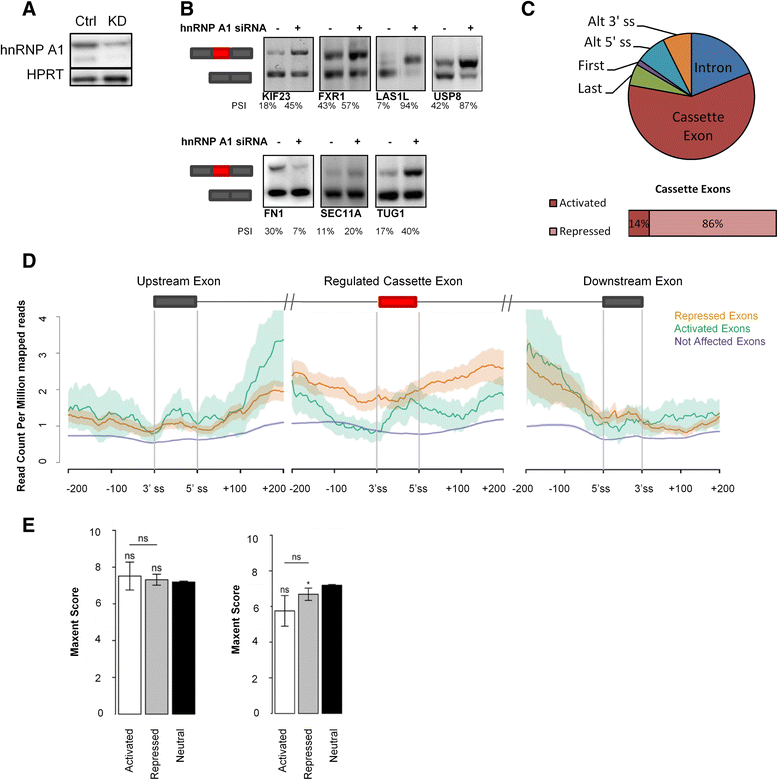
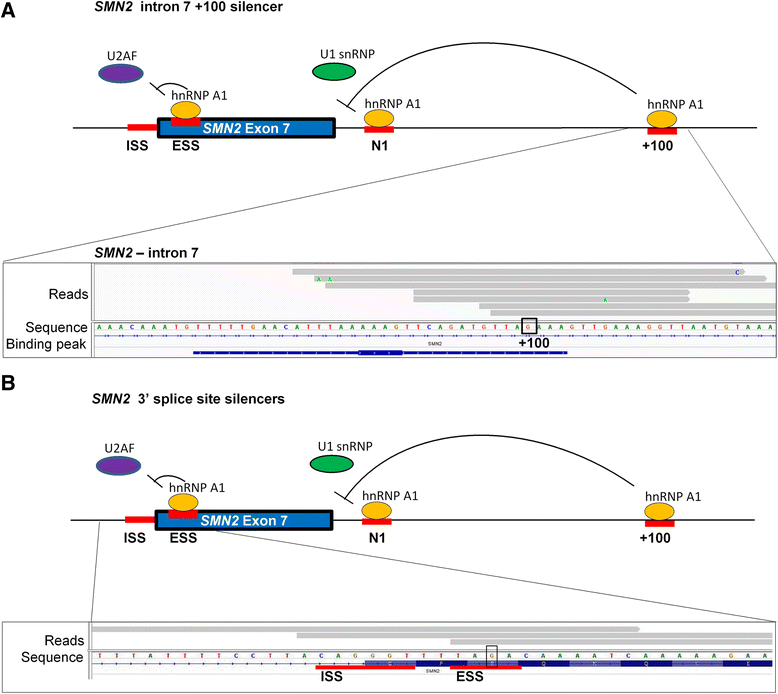
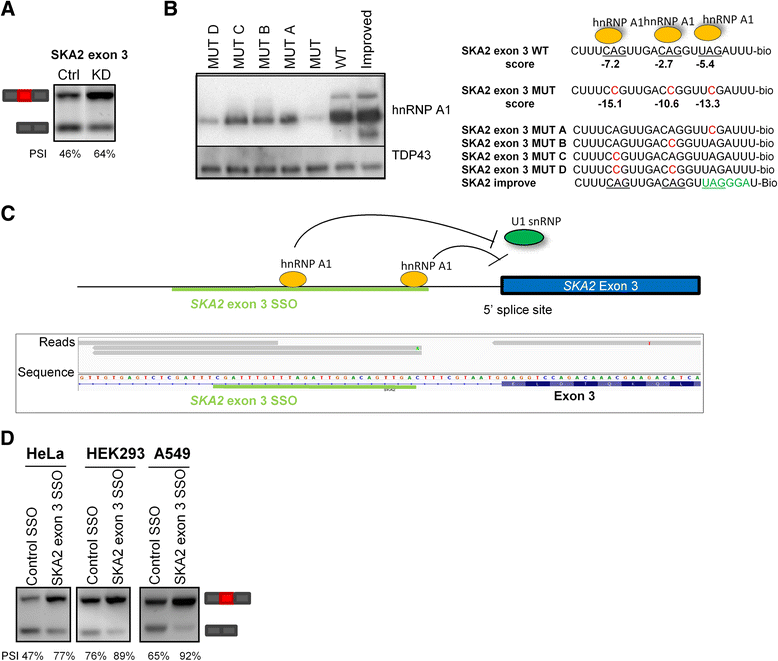
Results: Here, we used individual-nucleotide resolution crosslinking immunoprecipitation (iCLIP) to establish an in vivo binding map for the key splicing regulatory factor hnRNP A1 and to generate an hnRNP A1 consensus binding motif. We find that hnRNP A1 binding in proximal introns may be important for repressing exons. We show that inclusion of the alternative cassette exon 3 in SKA2 can be significantly increased by SSO-based treatment which blocks an iCLIP-identified hnRNP A1 binding site immediately downstream of the 5' splice site. Because pseudoexons are well suited as models for constitutive exons which have been inactivated by pathogenic mutations in SREs, we used a pseudoexon in MTRR as a model and showed that an iCLIP-identified hnRNP A1 binding site downstream of the 5' splice site can be blocked by SSOs to activate the exon.
Conclusions: The hnRNP A1 binding map can be used to identify potential targets for SSO-based therapy. Moreover, together with the hnRNP A1 consensus binding motif, the binding map may be used to predict whether disease-associated mutations and SNPs affect hnRNP A1 binding and eventually mRNA splicing.
Keywords: Alternative splicing; Cross-linking immunoprecipitation (CLIP); Pseudoexons; RNA-seq; Splicing silencer; Splicing splice-switching oligonucleotides (SSOs); Surface plasmon resonance imaging (SPRi); hnRNP A1; iCLIP.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

