Antibody-Dependent Enhancement of Dengue Virus Infection in Primary Human Macrophages; Balancing Higher Fusion against Antiviral Responses
- PMID: 27380892
- PMCID: PMC4933910
- DOI: 10.1038/srep29201
Antibody-Dependent Enhancement of Dengue Virus Infection in Primary Human Macrophages; Balancing Higher Fusion against Antiviral Responses
Abstract
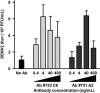
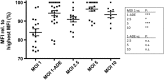
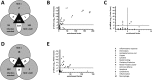
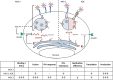
The dogma is that the human immune system protects us against pathogens. Yet, several viruses, like dengue virus, antagonize the hosts' antibodies to enhance their viral load and disease severity; a phenomenon called antibody-dependent enhancement of infection. This study offers novel insights in the molecular mechanism of antibody-mediated enhancement (ADE) of dengue virus infection in primary human macrophages. No differences were observed in the number of bound and internalized DENV particles following infection in the absence and presence of enhancing concentrations of antibodies. Yet, we did find an increase in membrane fusion activity during ADE of DENV infection. The higher fusion activity is coupled to a low antiviral response early in infection and subsequently a higher infection efficiency. Apparently, subtle enhancements early in the viral life cycle cascades into strong effects on infection, virus production and immune response. Importantly, and in contrast to other studies, the antibody-opsonized virus particles do not trigger immune suppression and remain sensitive to interferon. Additionally, this study gives insight in how human macrophages interact and respond to viral infections and the tight regulation thereof under various conditions of infection.
Figures


References
-
- Hawkes R. A. Enhancement of the Infectivity of Arboviruses by Specific Antisera Produced in Domestic Fowls. Aust. J. Exp. Biol. Med. Sci. 42, 465–482 (1964). - PubMed
-
- Halstead S. B. In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. J. Infect. Dis. 140, 527–533 (1979). - PubMed
-
- Sauter P. & Hober D. Mechanisms and results of the antibody-dependent enhancement of viral infections and role in the pathogenesis of coxsackievirus B-induced diseases. Microbes Infect. 11, 443–451 (2009). - PubMed
-
- Krilov L. R., Anderson L. J., Marcoux L., Bonagura V. R. & Wedgwood J. F. Antibody-mediated enhancement of respiratory syncytial virus infection in two monocyte/macrophage cell lines. J. Infect. Dis. 160, 777–782 (1989). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

