Self-assembly of c-myc DNA promoted by a single enantiomer ruthenium complex as a potential nuclear targeting gene carrier
- PMID: 27381008
- PMCID: PMC4933878
- DOI: 10.1038/srep28582
Self-assembly of c-myc DNA promoted by a single enantiomer ruthenium complex as a potential nuclear targeting gene carrier
Abstract
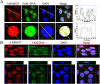
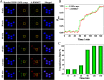
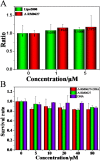
Gene therapy has long been limited in the clinic, due in part to the lack of safety and efficacy of the gene carrier. Herein, a single enantiomer ruthenium(II) complex, Λ-[Ru(bpy)2(p-BEPIP)](ClO4)2 (Λ-RM0627, bpy = 4,4'-bipyridine, p-BEPIP = 2-(4-phenylacetylenephenyl)imidazole [4,5f][1, 10] phenanthroline), has been synthesized and investigated as a potential gene carrier that targets the nucleus. In this report, it is shown that Λ-RM0627 promotes self-assembly of c-myc DNA to form a nanowire structure. Further studies showed that the nano-assembly of c-myc DNA that induced Λ-RM0627 could be efficiently taken up and enriched in the nuclei of HepG2 cells. After treatment of the nano-assembly of c-myc DNA with Λ-RM0627, over-expression of c-myc in HepG2 cells was observed. In summary, Λ-RM0627 played a key role in the transfer and release of c-myc into cells, which strongly indicates Λ-RM0627 as a potent carrier of c-myc DNA that targets the nucleus of tumor cells.
Figures



Similar articles
-
Chiral Ru(ii) complexes act as a potential non-viral gene carrier for directional transportation to the nucleus and cytoplasm.Metallomics. 2020 Apr 1;12(4):504-513. doi: 10.1039/c9mt00192a. Epub 2020 Feb 13. Metallomics. 2020. PMID: 32051986
-
Imaging Nuclei of MDA-MB-231 Breast Cancer Cells by Chiral Ruthenium(II) Complex Coordinated by 2-(4-Phenyacetylenephenyl)-1H-imidazo[4,5f][1,10]phenanthroline.Inorg Chem. 2016 Jun 6;55(11):5710-8. doi: 10.1021/acs.inorgchem.6b00824. Epub 2016 May 18. Inorg Chem. 2016. PMID: 27191197
-
Ruthenium(II) complexes as apoptosis inducers by stabilizing c-myc G-quadruplex DNA.Eur J Med Chem. 2014 Jun 10;80:316-24. doi: 10.1016/j.ejmech.2014.04.070. Epub 2014 Apr 25. Eur J Med Chem. 2014. PMID: 24793882
-
Microwave-assisted synthesis of ruthenium(II) complexes with alkynes as potential inhibitor by selectively recognizing c-myc G-quadruplex DNA.J Inorg Biochem. 2017 Nov;176:113-122. doi: 10.1016/j.jinorgbio.2017.08.005. Epub 2017 Aug 30. J Inorg Biochem. 2017. PMID: 28888786
-
Critical Overview of the Use of Ru(II) Polypyridyl Complexes as Photosensitizers in One-Photon and Two-Photon Photodynamic Therapy.Acc Chem Res. 2017 Nov 21;50(11):2727-2736. doi: 10.1021/acs.accounts.7b00180. Epub 2017 Oct 23. Acc Chem Res. 2017. PMID: 29058879 Review.
Cited by
-
DNA-based nanosized functional ruthenium(II) complex as potential phosphorescent probe to track tumor cells.Mol Divers. 2025 Apr;29(2):1161-1173. doi: 10.1007/s11030-024-10898-6. Epub 2024 Jun 15. Mol Divers. 2025. PMID: 38878213
-
Shining New Light on Biological Systems: Luminescent Transition Metal Complexes for Bioimaging and Biosensing Applications.Chem Rev. 2024 Aug 14;124(15):8825-9014. doi: 10.1021/acs.chemrev.3c00629. Epub 2024 Jul 25. Chem Rev. 2024. PMID: 39052606 Free PMC article. Review.
-
Preparation of Ru(ii)@oligonucleotide nanosized polymers as potential tumor-imaging luminescent probes.RSC Adv. 2018 Aug 30;8(53):30573-30581. doi: 10.1039/c8ra05454a. eCollection 2018 Aug 24. RSC Adv. 2018. PMID: 35546841 Free PMC article.
References
-
- Leboulch P. Gene therapy: primed for take-off. Nature 500, 280–282 (2013). - PubMed
-
- Korpisalo P. et al.. Capillary enlargement, not sprouting angiogenesis, determines beneficial therapeutic effects and side effects of angiogenic gene therapy. Eur. Heart J. 32, 1664–1672 (2011). - PubMed
-
- Yoo J. Y. et al.. Tumor suppression by apoptotic and anti-angiogenic effects of mortalin-targeting adeno-oncolytic virus. J. Gene Med. 12, 586–595 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

