The mRNA-bound proteome of the human malaria parasite Plasmodium falciparum
- PMID: 27381095
- PMCID: PMC4933991
- DOI: 10.1186/s13059-016-1014-0
The mRNA-bound proteome of the human malaria parasite Plasmodium falciparum
Abstract
Background: Gene expression is controlled at multiple levels, including transcription, stability, translation, and degradation. Over the years, it has become apparent that Plasmodium falciparum exerts limited transcriptional control of gene expression, while at least part of Plasmodium's genome is controlled by post-transcriptional mechanisms. To generate insights into the mechanisms that regulate gene expression at the post-transcriptional level, we undertook complementary computational, comparative genomics, and experimental approaches to identify and characterize mRNA-binding proteins (mRBPs) in P. falciparum.
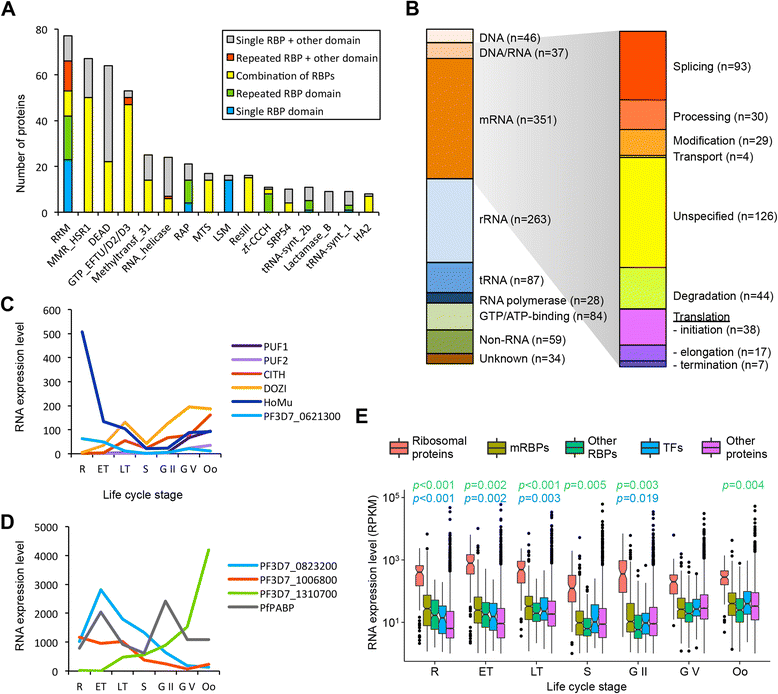
Results: Close to 1000 RNA-binding proteins are identified by hidden Markov model searches, of which mRBPs encompass a relatively large proportion of the parasite proteome as compared to other eukaryotes. Several abundant mRNA-binding domains are enriched in apicomplexan parasites, while strong depletion of mRNA-binding domains involved in RNA degradation is observed. Next, we experimentally capture 199 proteins that interact with mRNA during the blood stages, 64 of which with high confidence. These captured mRBPs show a significant overlap with the in silico identified candidate RBPs (p < 0.0001). Among the experimentally validated mRBPs are many known translational regulators active in other stages of the parasite's life cycle, such as DOZI, CITH, PfCELF2, Musashi, and PfAlba1-4. Finally, we also detect several proteins with an RNA-binding domain abundant in Apicomplexans (RAP domain) that is almost exclusively found in apicomplexan parasites.
Conclusions: Collectively, our results provide the most complete comparative genomics and experimental analysis of mRBPs in P. falciparum. A better understanding of these regulatory proteins will not only give insight into the intricate parasite life cycle but may also provide targets for novel therapeutic strategies.
Keywords: Gene expression; Post-transcriptional regulation; Protein domains; RNA-binding proteins; Translation.
Figures



References
-
- WHO. The World Malaria Report. 2015. http://www.who.int/malaria/publications/world-malaria-report-2015/report....

