A crystal-clear zebrafish for in vivo imaging
- PMID: 27381182
- PMCID: PMC4933947
- DOI: 10.1038/srep29490
A crystal-clear zebrafish for in vivo imaging
Abstract
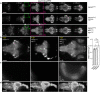
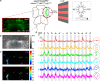
The larval zebrafish (Danio rerio) is an excellent vertebrate model for in vivo imaging of biological phenomena at subcellular, cellular and systems levels. However, the optical accessibility of highly pigmented tissues, like the eyes, is limited even in this animal model. Typical strategies to improve the transparency of zebrafish larvae require the use of either highly toxic chemical compounds (e.g. 1-phenyl-2-thiourea, PTU) or pigmentation mutant strains (e.g. casper mutant). To date none of these strategies produce normally behaving larvae that are transparent in both the body and the eyes. Here we present crystal, an optically clear zebrafish mutant obtained by combining different viable mutations affecting skin pigmentation. Compared to the previously described combinatorial mutant casper, the crystal mutant lacks pigmentation also in the retinal pigment epithelium, therefore enabling optical access to the eyes. Unlike PTU-treated animals, crystal larvae are able to perform visually guided behaviours, such as the optomotor response, as efficiently as wild type larvae. To validate the in vivo application of crystal larvae, we performed whole-brain light-sheet imaging and two-photon calcium imaging of neural activity in the retina. In conclusion, this novel combinatorial pigmentation mutant represents an ideal vertebrate tool for completely unobstructed structural and functional in vivo investigations of biological processes, particularly when imaging tissues inside or between the eyes.
Figures


References
-
- Jacques S. L. Optical properties of biological tissues: a review. Phys Med Biol 58, R37–R61 (2013). - PubMed
-
- Pawley J. B. Handbook of biological confocal microscopy. (Springer, 2005).
-
- Singh A. P. & Nusslein-Volhard C. Zebrafish stripes as a model for vertebrate colour pattern formation. Curr Biol 25, R81–R92 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

