Retinal determination genes coordinate neuroepithelial specification and neurogenesis modes in the Drosophila optic lobe
- PMID: 27381228
- PMCID: PMC4958324
- DOI: 10.1242/dev.135004
Retinal determination genes coordinate neuroepithelial specification and neurogenesis modes in the Drosophila optic lobe
Abstract
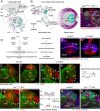
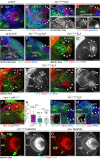
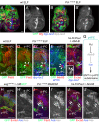
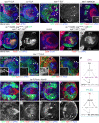
Differences in neuroepithelial patterning and neurogenesis modes contribute to area-specific diversifications of neural circuits. In the Drosophila visual system, two neuroepithelia, the outer (OPC) and inner (IPC) proliferation centers, generate neuron subtypes for four ganglia in several ways. Whereas neuroepithelial cells in the medial OPC directly convert into neuroblasts, in an IPC subdomain they generate migratory progenitors by epithelial-mesenchymal transition that mature into neuroblasts in a second proliferative zone. The molecular mechanisms that regulate the identity of these neuroepithelia, including their neurogenesis modes, remain poorly understood. Analysis of Polycomblike revealed that loss of Polycomb group-mediated repression of the Hox gene Abdominal-B (Abd-B) caused the transformation of OPC to IPC neuroepithelial identity. This suggests that the neuroepithelial default state is IPC-like, whereas OPC identity is derived. Ectopic Abd-B blocks expression of the highly conserved retinal determination gene network members Eyes absent (Eya), Sine oculis (So) and Homothorax (Hth). These factors are essential for OPC specification and neurogenesis control. Finally, eya and so are also sufficient to confer OPC-like identity, and, in parallel with hth, the OPC-specific neurogenesis mode on the IPC.
Keywords: Drosophila; Neuroepithelial specification; Neurogenesis; Polycomblike; Retinal determination genes; Visual system.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

