The Chlamydia-Secreted Protease CPAF Promotes Chlamydial Survival in the Mouse Lower Genital Tract
- PMID: 27382018
- PMCID: PMC4995919
- DOI: 10.1128/IAI.00280-16
The Chlamydia-Secreted Protease CPAF Promotes Chlamydial Survival in the Mouse Lower Genital Tract
Abstract
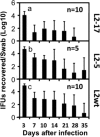
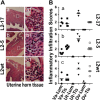
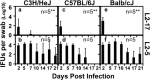
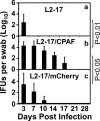
Despite the extensive in vitro characterization of CPAF (chlamydial protease/proteasome-like activity factor), its role in chlamydial infection and pathogenesis remains unclear. We now report that a Chlamydia trachomatis strain deficient in expression of CPAF (L2-17) is no longer able to establish a successful infection in the mouse lower genital tract following an intravaginal inoculation. The L2-17 organisms were cleared from the mouse lower genital tract within a few days, while a CPAF-sufficient C. trachomatis strain (L2-5) survived in the lower genital tract for more than 3 weeks. However, both the L2-17 and L2-5 organisms maintained robust infection courses that lasted up to 4 weeks when they were directly delivered into the mouse upper genital tract. The CPAF-dependent chlamydial survival in the lower genital tract was confirmed in multiple strains of mice. Thus, we have demonstrated a critical role of CPAF in promoting C. trachomatis survival in the mouse lower genital tracts. It will be interesting to further investigate the mechanisms of the CPAF-dependent chlamydial pathogenicity.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures


Similar articles
-
Chlamydia trachomatis outer membrane complex protein B (OmcB) is processed by the protease CPAF.J Bacteriol. 2013 Mar;195(5):951-7. doi: 10.1128/JB.02087-12. Epub 2012 Dec 7. J Bacteriol. 2013. PMID: 23222729 Free PMC article.
-
Human antibody responses to a Chlamydia-secreted protease factor.Infect Immun. 2004 Dec;72(12):7164-71. doi: 10.1128/IAI.72.12.7164-7171.2004. Infect Immun. 2004. PMID: 15557641 Free PMC article.
-
Chlamydial protease-like activity factor induces protective immunity against genital chlamydial infection in transgenic mice that express the human HLA-DR4 allele.Infect Immun. 2006 Dec;74(12):6722-9. doi: 10.1128/IAI.01119-06. Epub 2006 Oct 2. Infect Immun. 2006. PMID: 17015458 Free PMC article.
-
Chlamydial protease-like activity factor--insights into immunity and vaccine development.J Reprod Immunol. 2009 Dec;83(1-2):179-84. doi: 10.1016/j.jri.2009.05.007. Epub 2009 Oct 23. J Reprod Immunol. 2009. PMID: 19853923 Free PMC article. Review.
-
[Chlamydia trachomatis proteasome protein as one of the significant pathogenicity factors of exciter].Mol Gen Mikrobiol Virusol. 2014;(2):3-8. Mol Gen Mikrobiol Virusol. 2014. PMID: 25080811 Review. Russian.
Cited by
-
Suppression of Chlamydial Pathogenicity by Nonspecific CD8+ T Lymphocytes.Infect Immun. 2020 Sep 18;88(10):e00315-20. doi: 10.1128/IAI.00315-20. Print 2020 Sep 18. Infect Immun. 2020. PMID: 32747602 Free PMC article.
-
Pathogenic and Protective Roles of Neutrophils in Chlamydia trachomatis Infection.Pathogens. 2025 Jan 23;14(2):112. doi: 10.3390/pathogens14020112. Pathogens. 2025. PMID: 40005489 Free PMC article. Review.
-
Chlamydia Spreading from the Genital Tract to the Gastrointestinal Tract - A Two-Hit Hypothesis.Trends Microbiol. 2018 Jul;26(7):611-623. doi: 10.1016/j.tim.2017.12.002. Epub 2017 Dec 27. Trends Microbiol. 2018. PMID: 29289422 Free PMC article. Review.
-
Chlamydial Protease-Like Activity Factor and Type III Secreted Effectors Cooperate in Inhibition of p65 Nuclear Translocation.mBio. 2016 Sep 27;7(5):e01427-16. doi: 10.1128/mBio.01427-16. mBio. 2016. PMID: 27677792 Free PMC article.
-
Contributions of diverse models of the female reproductive tract to the study of Chlamydia trachomatis-host interactions.Curr Opin Microbiol. 2024 Feb;77:102416. doi: 10.1016/j.mib.2023.102416. Epub 2023 Dec 15. Curr Opin Microbiol. 2024. PMID: 38103413 Free PMC article. Review.
References
-
- Sharma M, Sethi S, Daftari S, Malhotra S. 2003. Evidence of chlamydial infection in infertile women with fallopian tube obstruction. Indian J Pathol Microbiol 46:680–683. - PubMed
-
- Sturdevant GL, Kari L, Gardner DJ, Olivares-Zavaleta N, Randall LB, Whitmire WM, Carlson JH, Goheen MM, Selleck EM, Martens C, Caldwell HD. 2010. Frameshift mutations in a single novel virulence factor alter the in vivo pathogenicity of Chlamydia trachomatis for the female murine genital tract. Infect Immun 78:3660–3668. doi:10.1128/IAI.00386-10. - DOI - PMC - PubMed
-
- Olivares-Zavaleta N, Whitmire W, Gardner D, Caldwell HD. 2010. Immunization with the attenuated plasmidless Chlamydia trachomatis L2(25667R) strain provides partial protection in a murine model of female genitourinary tract infection. Vaccine 28:1454–1462. doi:10.1016/j.vaccine.2009.11.073. - DOI - PMC - PubMed
-
- Sigar IM, Schripsema JH, Wang Y, Clarke IN, Cutcliffe LT, Seth-Smith HM, Thomson NR, Bjartling C, Unemo M, Persson K, Ramsey KH. 2014. Plasmid deficiency in urogenital isolates of Chlamydia trachomatis reduces infectivity and virulence in a mouse model. Pathog Dis 70:61–69. doi:10.1111/2049-632X.12086. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

