Lenalidomide in Relapsed or Refractory Diffuse Large B-Cell Lymphoma: Is It a Valid Treatment Option?
- PMID: 27382029
- PMCID: PMC5016065
- DOI: 10.1634/theoncologist.2016-0103
Lenalidomide in Relapsed or Refractory Diffuse Large B-Cell Lymphoma: Is It a Valid Treatment Option?
Abstract
Background: Despite the advent of new treatment strategies, many patients with diffuse large B-cell lymphoma (DLBCL) relapse or die of the disease. Prospective clinical trials have demonstrated that lenalidomide is an effective and safe treatment option, especially for non-germinal center B-cell (non-GCB) DLBCL. However, routine clinical data are lacking, which is why we provide the results of the so-far largest relapsed/refractory (R/R) DLBCL real-life analysis.
Methods: We retrospectively assessed 123 R/R DLBCL patients who received either 15 or 25 mg/day of lenalidomide from January 2006 to January 2015.
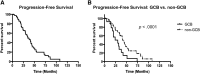
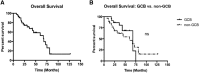
Results: During a median follow-up period of 4.5 years, complete remission was achieved in 32% and a partial remission in 33% non-GCB patients compared with 0% and 3% in the GCB group (p < .001 and .001, respectively), with median response durations of 15 and 5 months, respectively (p < .001). Lenalidomide at 25 mg was superior to 15 mg in terms of response (complete remission 21% and partial remission 23% vs. 0% and 8%; p = .007 and .05) and median response duration (10 vs. 4 months; p = .03). Toxicity was limited and reversible. Median progression-free survival differed between non-GCB and GCB patients (37 vs. 30 months; p < .001) and between the two dosages (24 vs. 34 months; p = .002). However, overall survival was similar between the subgroups (38-42 months).
Conclusion: We provide evidence that lenalidomide is a valid treatment option for R/R DLBCL, with limited and reversible toxicity, and is more efficient in non-GCB DLBCL and at higher doses.
Implications for practice: Despite the advent of new treatment strategies, many patients with diffuse large B-cell lymphoma (DLBCL) relapse or die of the disease; hence, novel therapeutic approaches are urgently needed. This study confirms that lenalidomide is a valid and well-tolerated treatment option for relapsed/refractory (R/R) DLBCL. Superior outcomes were observed in non-germinal center B-cell (GCB) DLBCL, probably because of inhibition of the nuclear factor-κB pathway. Similarly, high drug doses resulted in greater clinical benefits. Overall, lenalidomide is a suitable therapeutic option for R/R DLBCL, especially in non-GCB DLBCL, and 25 mg/day dosing should be preferred.
Keywords: Diffuse large B-cell lymphoma; Immunomodulatory drug; Lenalidomide; Relapsed/refractory lymphoma.
©AlphaMed Press.
Conflict of interest statement
of potential conflicts of interest may be found at the end of this article.
Figures
References
-
- Al-Hamadani M, Habermann TM, Cerhan JR, et al. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: A longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am J Hematol. 2015;90:790–795. - PubMed
-
- Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

