Control of skeletal muscle atrophy in response to disuse: clinical/preclinical contentions and fallacies of evidence
- PMID: 27382036
- PMCID: PMC5142005
- DOI: 10.1152/ajpendo.00257.2016
Control of skeletal muscle atrophy in response to disuse: clinical/preclinical contentions and fallacies of evidence
Abstract
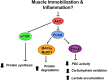
Muscle wasting resulting wholly or in part from disuse represents a serious medical complication that, when prolonged, can increase morbidity and mortality. Although much knowledge has been gained over the past half century, the underlying etiology by which disuse alters muscle proteostasis remains enigmatic. Multidisciplinary and novel methodologies are needed to fill gaps and overcome barriers to improved patient care. The present review highlights seminal concepts from a symposium at Experimental Biology 2016. These proceedings focus on 1) the role of insulin resistance in mediating disuse-induced changes in muscle protein synthesis (MPS) and breakdown (MPB), as well as cross-talk between carbohydrate and protein metabolism; 2) the relative importance of MPS/MPB in mediating involuntary muscle loss in humans and animals; 3) interpretative limitations associated with MPS/MPB "markers," e.g., MuRF1/MAFbx mRNA; and finally, 4) how OMIC technologies can be leveraged to identify molecular pathways (e.g., ATF4, p53, p21) mediating disuse atrophy. This perspective deals primarily with "simple atrophy" due to unloading. Nonetheless, it is likely that disuse is a pervasive contributor to muscle wasting associated with catabolic disease-related atrophy (i.e., due to associated sedentary behaviour of disease burden). Key knowledge gaps and challenges are identified to stimulate discussion and identify opportunities for translational research. Data from animal and human studies highlight both similarities and differences. Integrated preclinical and clinical research is encouraged to better understand the metabolic and molecular underpinnings and translational relevance,for disuse atrophy. These approaches are crucial to clinically prevent or reverse muscle atrophy, thereby reestablishing homeostasis and recovery.
Keywords: activating transcription factor 4; disuse atrophy; muscle ring finger 1; protein degradation; protein synthesis.
Copyright © 2016 the American Physiological Society.
Figures



References
-
- Bienso RS, Ringholm S, Kiilerich K, Aachmann-Andersen NJ, Krogh-Madsen R, Guerra B, Plomgaard P, van Hall G, Treebak JT, Saltin B, Lundby C, Calbet JA, Pilegaard H, Wojtaszewski JF. GLUT4 and glycogen synthase are key players in bed rest-induced insulin resistance. Diabetes 61: 1090–1099, 2012. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

