BRAF Mutation Correlates With High-Risk Langerhans Cell Histiocytosis and Increased Resistance to First-Line Therapy
- PMID: 27382093
- PMCID: PMC5321082
- DOI: 10.1200/JCO.2015.65.9508
BRAF Mutation Correlates With High-Risk Langerhans Cell Histiocytosis and Increased Resistance to First-Line Therapy
Abstract
Purpose: Langerhans cell histiocytosis (LCH) is an inflammatory myeloid neoplasia with a broad spectrum of clinical manifestations and outcomes in children. The somatic BRAF(V600E) mutation occurs frequently, but clinical significance remains to be determined.
Patients and methods: BRAF(V600E) mutation was investigated in a French LCH cohort. We analyzed associations between mutation status and clinical presentation, extent of disease, reactivation rate, response to therapy, and long-term permanent sequelae.
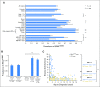
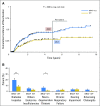
Results: Among 315 patients with successfully determined BRAF status, 173 (54.6%) carried a BRAF(V600E) mutation. Patients with BRAF(V600E) manifested more severe disease than did those with wild-type BRAF. Patients with BRAF(V600E) comprised 87.8% of patients (43 of 49) with multisystem LCH with risk organ involvement (liver, spleen, hematology), 68.6% of patients (35 of 51) with multisystem LCH without risk organ involvement, 43.9% of patients (86 of 196) with single-system LCH, and 42.1% of patients (8 of 19) with lung-involved LCH (P < .001). BRAF(V600E) mutation was also associated with organ involvement that could lead to permanent, irreversible damage, such as neurologic (75%) and pituitary (72.9%) injuries. Compared with patients with wild-type BRAF, patients with BRAF(V600E) more commonly displayed resistance to combined vinblastine and corticosteroid therapy (21.9% v 3.3%; P = .001), showed a higher reactivation rate (5-year reactivation rate, 42.8% v 28.1%; P = .006), and had more permanent, long-term consequences from disease or treatment (27.9% v 12.6%; P = .001).
Conclusion: In children with LCH, BRAF(V600E) mutation was associated with high-risk features, permanent injury, and poor short-term response to chemotherapy. Further population-based studies should be undertaken to confirm our observations and to assess the impact of BRAF inhibitors for this subgroup of patients who may benefit from targeted therapy.
© 2016 by American Society of Clinical Oncology.
Conflict of interest statement
Authors’ disclosures of potential conflicts of interest are found in the article online at
Figures
References
-
- Egeler RM, van Halteren AGS, Hogendoorn PCW, et al. Langerhans cell histiocytosis: Fascinating dynamics of the dendritic cell-macrophage lineage. Immunol Rev. 2010;234:213–232. - PubMed
-
- Gadner H, Grois N, Pötschger U, et al. Improved outcome in multisystem Langerhans cell histiocytosis is associated with therapy intensification. Blood. 2008;111:2556–2562. - PubMed
-
- Haupt R, Nanduri V, Calevo MG, et al. Permanent consequences in Langerhans cell histiocytosis patients: A pilot study from the Histiocyte Society-Late Effects Study Group. Pediatr Blood Cancer. 2004;42:438–444. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

