Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose
- PMID: 27382155
- PMCID: PMC4961123
- DOI: 10.1073/pnas.1600299113
Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose
Erratum in
-
Correction for Chahal et al., Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose.Proc Natl Acad Sci U S A. 2016 Aug 30;113(35):E5250. doi: 10.1073/pnas.1612792113. Epub 2016 Aug 22. Proc Natl Acad Sci U S A. 2016. PMID: 27551066 Free PMC article. No abstract available.
Abstract
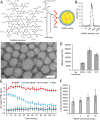
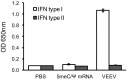
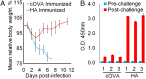
Vaccines have had broad medical impact, but existing vaccine technologies and production methods are limited in their ability to respond rapidly to evolving and emerging pathogens, or sudden outbreaks. Here, we develop a rapid-response, fully synthetic, single-dose, adjuvant-free dendrimer nanoparticle vaccine platform wherein antigens are encoded by encapsulated mRNA replicons. To our knowledge, this system is the first capable of generating protective immunity against a broad spectrum of lethal pathogen challenges, including H1N1 influenza, Toxoplasma gondii, and Ebola virus. The vaccine can be formed with multiple antigen-expressing replicons, and is capable of eliciting both CD8(+) T-cell and antibody responses. The ability to generate viable, contaminant-free vaccines within days, to single or multiple antigens, may have broad utility for a range of diseases.
Keywords: nanoparticle; parasites; replicon; vaccine platform; viruses.
Conflict of interest statement
Conflict of interest statement: A patent has been filed for the nanoparticle vaccine by J.S.C., O.F.K., R.L., H.L.P., and D.G.A.
Figures







References
-
- Arnon R, Ben-Yedidia T. Old and new vaccine approaches. Int Immunopharmacol. 2003;3(8):1195–1204. - PubMed
-
- Azad N, Rojanasakul Y. Vaccine delivery—Current trends and future. Curr Drug Deliv. 2006;3(2):137–146. - PubMed
-
- Pittman PR. Aluminum-containing vaccine associated adverse events: Role of route of administration and gender. Vaccine. 2002;20(Suppl 3):S48–S50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

