pH-degradable imidazoquinoline-ligated nanogels for lymph node-focused immune activation
- PMID: 27382168
- PMCID: PMC4961133
- DOI: 10.1073/pnas.1600816113
pH-degradable imidazoquinoline-ligated nanogels for lymph node-focused immune activation
Abstract
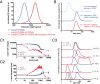
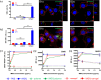
Agonists of Toll-like receptors (TLRs) are potent activators of the innate immune system and hold promise as vaccine adjuvant and for anticancer immunotherapy. Unfortunately, in soluble form they readily enter systemic circulation and cause systemic inflammatory toxicity. Here we demonstrate that by covalent ligation of a small-molecule imidazoquinoline-based TLR7/8 agonist to 50-nm-sized degradable polymeric nanogels the potency of the agonist to activate TLR7/8 in in vitro cultured dendritic cells is largely retained. Importantly, imidazoquinoline-ligated nanogels focused the in vivo immune activation on the draining lymph nodes while dramatically reducing systemic inflammation. Mechanistic studies revealed a prevalent passive diffusion of the nanogels to the draining lymph node. Moreover, immunization studies in mice have shown that relative to soluble TLR7/8 agonist, imidazoquinoline-ligated nanogels induce superior antibody and T-cell responses against a tuberculosis antigen. This approach opens possibilities to enhance the therapeutic benefit of small-molecule TLR agonist for a variety of applications.
Keywords: Toll-like receptor; dendritic cells; lymph node; nanotechnology; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303(5663):1529–1531. - PubMed
-
- Hemmi H, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3(2):196–200. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

