Temperate phages both mediate and drive adaptive evolution in pathogen biofilms
- PMID: 27382184
- PMCID: PMC4961188
- DOI: 10.1073/pnas.1520056113
Temperate phages both mediate and drive adaptive evolution in pathogen biofilms
Abstract
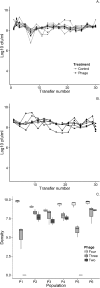
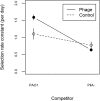
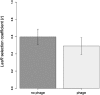
Temperate phages drive genomic diversification in bacterial pathogens. Phage-derived sequences are more common in pathogenic than nonpathogenic taxa and are associated with changes in pathogen virulence. High abundance and mobilization of temperate phages within hosts suggests that temperate phages could promote within-host evolution of bacterial pathogens. However, their role in pathogen evolution has not been experimentally tested. We experimentally evolved replicate populations of Pseudomonas aeruginosa with or without a community of three temperate phages active in cystic fibrosis (CF) lung infections, including the transposable phage, ɸ4, which is closely related to phage D3112. Populations grew as free-floating biofilms in artificial sputum medium, mimicking sputum of CF lungs where P. aeruginosa is an important pathogen and undergoes evolutionary adaptation and diversification during chronic infection. Although bacterial populations adapted to the biofilm environment in both treatments, population genomic analysis revealed that phages altered both the trajectory and mode of evolution. Populations evolving with phages exhibited a greater degree of parallel evolution and faster selective sweeps than populations without phages. Phage ɸ4 integrated randomly into the bacterial chromosome, but integrations into motility-associated genes and regulators of quorum sensing systems essential for virulence were selected in parallel, strongly suggesting that these insertional inactivation mutations were adaptive. Temperate phages, and in particular transposable phages, are therefore likely to facilitate adaptive evolution of bacterial pathogens within hosts.
Keywords: Pseudomonas aeruginosa; bacteriophage; cystic fibrosis; experimental evolution; mobile genetic element.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

