C-Terminal Alpha-1 Antitrypsin Peptide: A New Sepsis Biomarker with Immunomodulatory Function
- PMID: 27382189
- PMCID: PMC4921625
- DOI: 10.1155/2016/6129437
C-Terminal Alpha-1 Antitrypsin Peptide: A New Sepsis Biomarker with Immunomodulatory Function
Abstract
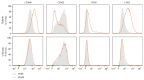
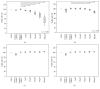
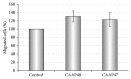
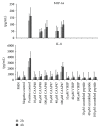
Systemic inflammatory response syndrome (SIRS) is a life threatening condition and the leading cause of death in intensive care units. Although single aspects of pathophysiology have been described in detail, numerous unknown mediators contribute to the progression of this complex disease. The aim of this study was to elucidate the pathophysiological role of CAAP48, a C-terminal alpha-1 antitrypsin fragment, that we found to be elevated in septic patients and to apply this peptide as diagnostic marker for infectious and noninfectious etiologies of SIRS. Incubation of human polymorphonuclear neutrophils with synthetic CAAP48, the SNP-variant CAAP47, and several control peptides revealed intense neutrophil activation, induction of neutrophil chemotaxis, reduction of neutrophil viability, and release of cytokines. We determined the abundance of CAAP48 in patients with severe sepsis, severe SIRS of noninfectious origin, and viral infection. CAAP48 levels were 3-4-fold higher in patients with sepsis compared to SIRS of noninfectious origin and allowed discrimination of those patients with high sensitivity and specificity. Our results suggest that CAAP48 is a promising discriminatory sepsis biomarker with immunomodulatory functions, particularly on human neutrophils, supporting its important role in the host response and pathophysiology of sepsis.
Figures




References
-
- Tilg H., Vannier E., Vachino G., Dinarello C. A., Mier J. W. Antiinflammatory properties of hepatic acute phase proteins: preferential induction of interleukin 1 (IL-1) receptor antagonist over IL-1β synthesis by human peripheral blood mononuclear cells. Journal of Experimental Medicine. 1993;178(5):1629–1636. doi: 10.1084/jem.178.5.1629. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

