Double-blind, randomized, double-dummy clinical trial comparing the efficacy of ketorolac trometamol and naproxen for acute low back pain
- PMID: 27382251
- PMCID: PMC4918732
- DOI: 10.2147/DDDT.S97756
Double-blind, randomized, double-dummy clinical trial comparing the efficacy of ketorolac trometamol and naproxen for acute low back pain
Abstract
Background: Nonsteroidal anti-inflammatory drugs (NSAIDs) are the most common type of medication used in the treatment of acute pain. Ketorolac trometamol (KT) is a nonnarcotic, peripherally acting nonsteroidal anti-inflammatory drug with analgesic effects comparable to certain opioids.
Objective: The aim of this study was to compare the efficacy of KT and naproxen (NA) in the treatment of acute low back pain (LBP) of moderate-to-severe intensity.
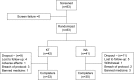
Patients and methods: In this 10-day, Phase III, randomized, double-blind, double-dummy, noninferiority trial, participants with acute LBP of moderate-to-severe intensity as determined through a visual analog scale (VAS) were randomly assigned in a 1:1 ratio to receive sublingual KT 10 mg three times daily or oral NA 250 mg three times daily. From the second to the fifth day of treatment, if patient had VAS >40 mm, increased dosage to four times per day was allowed. The primary end point was the reduction in LBP as measured by VAS. We also performed a post hoc superiority analysis.
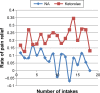
Results: KT was not inferior to NA for the reduction in LBP over 5 days of use as measured by VAS scores (P=0.608 for equality of variance; P=0.321 for equality of means) and by the Roland-Morris Disability Questionnaire (P=0.180 for equality of variance test; P=0.446 for equality of means) using 95% confidence intervals. The percentage of participants with improved pain relief 60 minutes after receiving the first dose was higher in the KT group (24.2%) than in the NA group (6.5%; P=0.049). The most common adverse effects were heartburn, nausea, and vomiting.
Conclusion: KT is not inferior in efficacy and delivers faster pain relief than NA.
Keywords: acute low back pain; ketorolac trometamol; naproxen; nonsteroidal anti-inflammatory drugs.
Figures
References
-
- Hills EC [webpage on the Internet] Mechanical Low Back Pain. 2014. [Accessed May 13, 2016]. [updated Aug 4, 2014]. Available from: http://emedicine.medscape.com/article/310353-followup.
-
- Ijzelenberg W, Burdorf A. Risk factors for musculoskeletal symptoms and ensuing health care use and sick leave. Spine. 2005;30(13):1550–1556. - PubMed
-
- Lötsch J, Geisslinger G. Current evidence for genetic modulation of the response to analgesics. Pain. 2006;121:1–5. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous