MUC16 contributes to the metastasis of pancreatic ductal adenocarcinoma through focal adhesion mediated signaling mechanism
- PMID: 27382435
- PMCID: PMC4918949
- DOI: 10.18632/genesandcancer.104
MUC16 contributes to the metastasis of pancreatic ductal adenocarcinoma through focal adhesion mediated signaling mechanism
Abstract
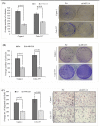
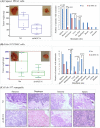
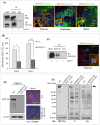
MUC16, a heavily glycosylated type-I transmembrane mucin is overexpressed in several cancers including pancreatic ductal adenocarcinoma (PDAC). Previously, we have shown that MUC16 is significantly overexpressed in human PDAC tissues. However, the functional consequences and its role in PDAC is poorly understood. Here, we show that MUC16 knockdown decreases PDAC cell proliferation, colony formation and migration in vitro. Also, MUC16 knockdown decreases the tumor formation and metastasis in orthotopic xenograft mouse model. Mechanistically, immunoprecipitation and immunofluorescence analyses confirms MUC16 interaction with galectin-3 and mesothelin in PDAC cells. Adhesion assay displayed decreased cell attachment of MUC16 knockdown cells with recombinant galectin-1 and galectin-3 protein. Further, CRISPR/Cas9-mediated MUC16 knockout cells show decreased tumor-associated carbohydrate antigens (T and Tn) in PDAC cells. Importantly, carbohydrate antigens were decreased in the region that corresponds to MUC16 and suggests for the decreased MUC16-galectin interactions. Co-immunoprecipitation also revealed a novel interaction between MUC16 and FAK in PDAC cells. Interestingly, we observed decreased expression of mesenchymal and increased expression of epithelial markers in MUC16-silenced cells. Additionally, MUC16 loss showed a decreased FAK-mediated Akt and ERK/MAPK activation. Altogether, these findings suggest that MUC16-focal adhesion signaling may play a critical role in facilitating PDAC growth and metastasis.
Keywords: CRISPR/Cas9; FAK; MUC16; metastasis; pancreatic cancer.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Chaturvedi P, Singh AP, Moniaux N, Senapati S, Chakraborty S, Meza JL, Batra SK. MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Mol Cancer Res. 2007;5:309–320. - PubMed
-
- Kohlgraf KG, Gawron AJ, Higashi M, Meza JL, Burdick MD, Kitajima S, Kelly DL, Caffrey TC, Hollingsworth MA. Contribution of the MUC1 tandem repeat and cytoplasmic tail to invasive and metastatic properties of a pancreatic cancer cell line. Cancer Res. 2003;63:5011–5020. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

