Hypertonicity: Pathophysiologic Concept and Experimental Studies
- PMID: 27382523
- PMCID: PMC4895078
- DOI: 10.7759/cureus.596
Hypertonicity: Pathophysiologic Concept and Experimental Studies
Abstract
Disturbances in tonicity (effective osmolarity) are the major clinical disorders affecting cell volume. Cell shrinking secondary to hypertonicity causes severe clinical manifestations and even death. Quantitative management of hypertonic disorders is based on formulas computing the volume of hypotonic fluids required to correct a given level of hypertonicity. These formulas have limitations. The major limitation of the predictive formulas is that they represent closed system calculations and have been tested in anuric animals. Consequently, the formulas do not account for ongoing fluid losses during development or treatment of the hypertonic disorders. In addition, early comparisons of serum osmolality changes predicted by these formulas and observed in animals infused with hypertonic solutions clearly demonstrated that hypertonicity creates new intracellular solutes causing rises in serum osmolality higher than those predicted by the formulas. The mechanisms and types of intracellular solutes generated by hypertonicity and the effects of the solutes have been studied extensively in recent times. The solutes accumulated intracellularly in hypertonic states have potentially major adverse effects on the outcomes of treatment of these states. When hypertonicity was produced by the infusion of hypertonic sodium chloride solutions, the predicted and observed changes in serum sodium concentration were equal. This finding justifies the use of the predictive formulas in the management of hypernatremic states.
Keywords: hypertonicity; osmolality; osmolarity; osmolytes; serum sodium concentration.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
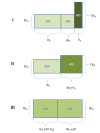

References
-
- Maffly RH. The Kidney, 1st edition. Vol. 1. Philadelphia: WB Saunders; 1976. The body fluids: Volume, composition, and clinical chemistry; pp. 65–103.
-
- Guyton AC, Hall JE. Human Physiology and Mechanisms of Disease, 6th Edition. Philadelphia: WB Saunders; 1996. The body fluid compartments; pp. 201–211.
-
- Water exchange. Peters JP. http://physrev.physiology.org/content/24/4/491.short Physiol Rev. 1944;24:491–531.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
