Control of Drug Dissolution Rate from Film Dosage Forms Containing Valsartan
- PMID: 27382640
- PMCID: PMC4897208
- DOI: 10.1155/2016/5135173
Control of Drug Dissolution Rate from Film Dosage Forms Containing Valsartan
Abstract
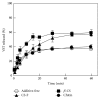
Film dosage forms (FDs) containing valsartan (VST), a popular antihypertensive drug, were prepared using a casting method with sodium alginate and other polysaccharides as the film base. Drug dissolution profiles of the FDs were investigated in limited medium. The FDs were 170-200 μm thick and were easy to handle. All FDs immediately swelled and disintegrated in the medium. About 23% of the VST incorporated into the FD prepared with 1.5% sodium alginate dissolved at 5 min. The initial dissolution rate of VST increased upon the addition of chitosan to the film base; this effect was not observed in the case of chitin. On the other hand, the rate apparently decreased upon modification with alginic acid. In addition, the solubility of VST in the dissolution medium was changed by the addition of chitosan or alginic acid. FDs prepared with polysaccharides are useful for simplifying the administration of drugs to patients, and the drug dissolution rate from FDs can be controlled by modification.
Figures



References
-
- Visser J. C., Dohmen W. M. C., Hinrichs W. L. J., Breitkreutz J., Frijlink H. W., Woerdenbag H. J. Quality by design approach for optimizing the formulation and physical properties of extemporaneously prepared orodispersible films. International Journal of Pharmaceutics. 2015;485(1-2):70–76. doi: 10.1016/j.ijpharm.2015.03.005. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

