Rab35 Functions in Axon Elongation Are Regulated by P53-Related Protein Kinase in a Mechanism That Involves Rab35 Protein Degradation and the Microtubule-Associated Protein 1B
- PMID: 27383602
- PMCID: PMC6705529
- DOI: 10.1523/JNEUROSCI.4064-15.2016
Rab35 Functions in Axon Elongation Are Regulated by P53-Related Protein Kinase in a Mechanism That Involves Rab35 Protein Degradation and the Microtubule-Associated Protein 1B
Abstract
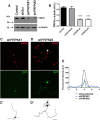
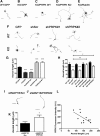
Rab35 is a key protein for cargo loading in the recycling endosome. In neuronal immortalized cells, Rab35 promotes neurite differentiation. Here we describe that Rab35 favors axon elongation in rat primary neurons in an activity-dependent manner. In addition, we show that the p53-related protein kinase (PRPK) negatively regulates axonal elongation by reducing Rab35 protein levels through the ubiquitin-proteasome degradation pathway. PRPK-induced Rab35 degradation is regulated by its interaction with microtubule-associated protein 1B (MAP1B), a microtubule stabilizing binding protein essential for axon elongation. Consistently, axon defects found in MAP1B knock-out neurons were reversed by Rab35 overexpression or PRPK inactivation suggesting an epistatic relationship among these proteins. These results define a novel mechanism to support axonal elongation, by which MAP1B prevents PRPK-induced Rab35 degradation. Such a mechanism allows Rab35-mediated axonal elongation and connects the regulation of actin dynamics with membrane trafficking. In addition, our study reveals for the first time that the ubiquitin-proteasome degradation pathway regulates a Rab GTPase.
Significance statement: Rab35 is required for axonal outgrowth. We define that its protein levels are negatively regulated by p53-related protein kinase (PRPK). We show that microtubule-associated protein 1B (MAP1B) interacts with PRPK, preventing PRPK-dependent Rab35 proteasome degradation. We demonstrate that Rab35 regulates Cdc42 activity in neurons. This is the first evidence showing that a Rab protein is regulated by degradation dependent on the ubiquitin-proteasome system.
Keywords: MAP1B; Rab35; axon development; p53-related protein kinase; ubiquitin proteosome.
Copyright © 2016 the authors 0270-6474/16/367298-16$15.00/0.
Figures




References
-
- Abe Y, Matsumoto S, Wei S, Nezu K, Miyoshi A, Kito K, Ueda N, Shigemoto K, Hitsumoto Y, Nikawa J, Enomoto Y. Cloning and characterization of a p53-related protein kinase expressed in interleukin-2-activated cytotoxic T-cells, epithelial tumor cell lines, and the testes. J Biol Chem. 2001;276:44003–44011. doi: 10.1074/jbc.M105669200. - DOI - PubMed
-
- Bouquet C, Soares S, von Boxberg Y, Ravaille-Veron M, Propst F, Nothias F. Microtubule-associated protein 1B controls directionality of growth cone migration and axonal branching in regeneration of adult dorsal root ganglia neurons. J Neurosci. 2004;24:7204–7213. doi: 10.1523/JNEUROSCI.2254-04.2004. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
