Efficient Inhibition of Hepatitis B Virus Infection by a preS1-binding Peptide
- PMID: 27384014
- PMCID: PMC4935942
- DOI: 10.1038/srep29391
Efficient Inhibition of Hepatitis B Virus Infection by a preS1-binding Peptide
Abstract
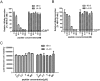
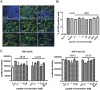
Entry inhibitors are promising novel antivirals against hepatitis B virus (HBV) infection. The existing potential entry inhibitors have targeted the cellular receptor(s). In this study, we aim to develop the first entry inhibitor that inhibits HBV infection via targeting viral particles. The preS1 segment of the large envelope glycoprotein of HBV is essential for virion attachment and infection. Previously, we obtained a preS1-binding short peptide B10 by screening a phage display peptide library using the N-terminal half of preS1 (residues 1 to 60, genotype C). We report here that by means of concatenation of B10, we identified a quadruple concatemer 4B10 that displayed a markedly increased preS1-binding activity. The main binding site of 4B10 in preS1 was mapped to the receptor binding enhancing region. 4B10 blocked HBV attachment to hepatic cells and inhibited HBV infection of primary human and tupaia hepatocytes at low nanomolar concentrations. The 4B10-mediated inhibition of HBV infection is specific as it did not inhibit the infection of vesicular stomatitis virus glycoprotein pseudotyped lentivirus or human immunodeficiency virus type 1. Moreover, 4B10 showed no binding activity to hepatic cells. In conclusion, we have identified 4B10 as a promising candidate for a novel class of HBV entry inhibitors.
Figures

Similar articles
-
Mapping of the hepatitis B virus attachment site by use of infection-inhibiting preS1 lipopeptides and tupaia hepatocytes.Gastroenterology. 2005 Jul;129(1):234-45. doi: 10.1053/j.gastro.2005.03.090. Gastroenterology. 2005. PMID: 16012950
-
Molecular mechanisms of hepatitis B virus entry inhibition by a bile acid derivative INT-767 binding to the preS1 region.Antiviral Res. 2025 Aug;240:106213. doi: 10.1016/j.antiviral.2025.106213. Epub 2025 Jun 11. Antiviral Res. 2025. PMID: 40513783
-
N-terminal myristoylation-dependent masking of neutralizing epitopes in the preS1 attachment site of hepatitis B virus.J Hepatol. 2011 Jul;55(1):29-37. doi: 10.1016/j.jhep.2010.10.019. Epub 2010 Nov 28. J Hepatol. 2011. PMID: 21145866
-
Strategies to inhibit entry of HBV and HDV into hepatocytes.Gastroenterology. 2014 Jul;147(1):48-64. doi: 10.1053/j.gastro.2014.04.030. Epub 2014 Apr 25. Gastroenterology. 2014. PMID: 24768844 Review.
-
Entry of hepatitis B virus: mechanism and new therapeutic target.Pathol Biol (Paris). 2010 Aug;58(4):301-7. doi: 10.1016/j.patbio.2010.04.001. Epub 2010 May 31. Pathol Biol (Paris). 2010. PMID: 20570056 Review.
Cited by
-
Recent Advances in Hepatitis B Treatment.Pharmaceuticals (Basel). 2021 May 1;14(5):417. doi: 10.3390/ph14050417. Pharmaceuticals (Basel). 2021. PMID: 34062711 Free PMC article. Review.
-
Antiviral Properties and Mechanism of Action Studies of the Hepatitis B Virus Capsid Assembly Modulator JNJ-56136379.Antimicrob Agents Chemother. 2020 Apr 21;64(5):e02439-19. doi: 10.1128/AAC.02439-19. Print 2020 Apr 21. Antimicrob Agents Chemother. 2020. PMID: 32094138 Free PMC article.
-
Drugs in Development for Hepatitis B.Drugs. 2017 Aug;77(12):1263-1280. doi: 10.1007/s40265-017-0769-2. Drugs. 2017. PMID: 28660478 Free PMC article. Review.
-
Non-Toxic Dimeric Peptides Derived from the Bothropstoxin-I Are Potent SARS-CoV-2 and Papain-like Protease Inhibitors.Molecules. 2021 Aug 12;26(16):4896. doi: 10.3390/molecules26164896. Molecules. 2021. PMID: 34443484 Free PMC article.
-
An update on antiviral antibody-based biopharmaceuticals.Int Immunopharmacol. 2020 Sep;86:106760. doi: 10.1016/j.intimp.2020.106760. Epub 2020 Jul 6. Int Immunopharmacol. 2020. PMID: 32645633 Free PMC article. Review.
References
-
- Ganem D. & Prince A. M. Hepatitis B virus infection-natural history and clinical consequences. N. Engl. J. Med. 350, 1118–1129 (2004). - PubMed
-
- Perrillo R. Benefits and risks of interferon therapy for hepatitis B. Hepatology 49, S103–S111 (2009). - PubMed
-
- Zoulim F. & Locarnini S. Hepatitis B virus resistance to nucleos(t)ide analogues. Gastroenterology 137, 1593–1608 (2009). - PubMed
-
- Zoulim F. Are novel combination therapies needed for chronic hepatitis B? Antiviral. Res. 96, 256–259 (2012). - PubMed
-
- Leroux-Roels G. et al. Prevention of hepatitis B infections: vaccination and its limitations. Acta Clin. Belg. 56, 209–219 (2001). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

