Sealing procedures for preterm prelabour rupture of membranes
- PMID: 27384151
- PMCID: PMC6457929
- DOI: 10.1002/14651858.CD010218.pub2
Sealing procedures for preterm prelabour rupture of membranes
Abstract
Background: Preterm prelabour rupture of the membranes (PPROM) complicates approximately 2% of pregnancies and can be either spontaneous or iatrogenic in nature. Complications of PPROM include prematurity, chorioamnionitis, neonatal sepsis, limb position defects, respiratory distress syndrome, pulmonary hypoplasia chronic lung disease, periventricular leukomalacia and intraventricular haemorrhage.A number of different sealing techniques have been employed which aim to restore a physical barrier against infection and encourage the re-accumulation of amniotic fluid. Routine use of sealants is currently not recommended due to a lack of sufficient evidence to support the safety and effectiveness of such interventions.
Objectives: To assess the effects of sealing techniques following PPROM against each other, or versus standard care (including no sealant), on maternal and neonatal outcomes.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 May 2016) and reference lists of retrieved studies.
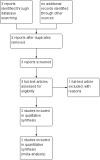
Selection criteria: Randomised and quasi-randomised controlled trials comparing different techniques for sealing preterm prelabour ruptured membranes. Cluster-randomised trials and trials using a cross-over design were not eligible for inclusion in this review. We planned to include abstracts when sufficient information was provided.
Data collection and analysis: Two review authors independently assessed trials for inclusion and assessed trial quality. Two review authors independently extracted data. Data were checked for accuracy.
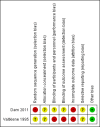
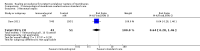
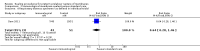
Main results: We included two studies (involving 141 women - with data from 124 women). We considered both studies as being at high risk of bias. Meta-analysis was not possible because the included studies examined different interventions (both in comparison with standard care) and reported on few, but different, outcomes. One study compared cervical adapter (mechanical sealing), and the other study examined an immunological membrane sealant. Neither of the included studies reported on this review's primary outcome of interest - perinatal mortality. Similarly, data were not reported for the majority of this review's secondary infant and maternal outcomes. Cervical adapter (mechanical sealing) versus standard care (one study, data from 35 participants)No data were reported for this review's primary outcome - perinatal mortality. Data were reported for few of this review's infant or maternal secondary outcomes.There was no clear difference between the mechanical sealing group and the standard care control in relation to the incidence of neonatal sepsis (risk ratio (RR) 1.19, 95% confidence interval (CI) 0.28 to 5.09 (very low-quality evidence)) or chorioamnionitis (RR 1.19, 95% CI 0.28 to 5.09 (very low-quality evidence)). Oral immunological membrane sealant versus standard care (one study, data from 94 participants)No data were available for perinatal mortality (this review's primary outcome) or for the majority of this review's infant and maternal secondary outcomes. Compared to standard care, the immunological membrane sealant was associated with a reduction in preterm birth less than 37 weeks (RR 0.48, 95% CI 0.34 to 0.68 (very low-quality evidence)) and a reduction in neonatal death (RR 0.38, 95% CI 0.19 to 0.75 (very low-quality evidence)). However, there was no clear difference between groups in terms of neonatal sepsis (RR 0.64, 95% CI 0.28 to 1.46 (very low-quality evidence)) or respiratory distress syndrome (RR 0.64, 95% CI 0.28 to 1.46 (very low-quality evidence)).
Authors' conclusions: There is insufficient evidence to evaluate sealing procedures for PPROM. There were no data relating to this review's primary outcome (perinatal mortality) and the majority of our infant and maternal secondary outcomes were not reported in the two included studies.There was limited evidence to suggest that an immunological membrane sealant was associated with a reduction in preterm birth at less than 37 weeks and neonatal death, but these results should be interpreted with caution as this is based on one small study, with a high risk of bias, and the intervention has not been tested in other studies.Although midtrimester PPROM is not a rare occurrence, there are only a small amount of published data addressing the benefits and risks of sealing procedures. Most of these studies are retrospective and cohort based and could therefore not be included in our data-analysis.This review highlights the paucity of prospective randomised trials in this area. Current evidence provides limited information both on effectiveness and safety for the interventions described. Given the paucity of high-quality data, we recommend that future research efforts focus on the conduct of randomised trials assessing the effect of promising interventions that have been only evaluated to date in cohort studies (e.g. amniopatch). Future trials should address outcomes including perinatal mortality, preterm birth, neonatal death, respiratory distress syndrome, neonatal sepsis and developmental delay. They should also evaluate maternal outcomes including sepsis, mode of delivery, length of hospital stay and emotional well-being.
Conflict of interest statement
Adele Crowley: none known.
Rosalie Grivell: none known..
Jodie Dodd: none known.
Figures




Update of
References
References to studies included in this review
Dam 2011 {published data only}
-
- Dam P, Somnath L, Parnamita B, Pallavi D. Role of amnioseal in premature rupture of membranes. Journal of Obstetrics and Gynaecology of India 2011;61(3):296‐300.
Vaitkiene 1995 {published data only}
-
- Vaitkiene D, Bergstrom S. Management of amniocentesis in women with oligohydramnios due to membrane rupture: evaluation of a cervical adapter. Gynecologic and Obstetric Investigation 1995;40:28‐31. - PubMed
References to studies excluded from this review
Kubo 1991 {published data only}
-
- Kubo T, Taniguchi K, Hamada T, Tsukahara Y, Sagara Y. New useful management of fibrin adhesion for premature rupture of the membranes during the second trimester of pregnancy. Journal of Perinatal Medicine 1991;19(Suppl 2):246.
Additional references
Behzad 1994
-
- Behzad F, Dickinson MR, Charlton A, Aplin JD. Brief communication: sliding displacement of amnion and chorion following controlled laser wounding suggests a mechanism for short term sealing of ruptured membranes. Placenta 1994;15:775‐8. - PubMed
Bengtson 1989
-
- Bengtson J, VanMarter L, Barss V. Pregnancy outcome after premature rupture of the membranes at or before 26 weeks' gestation. Obstetrics and Gynecology 1989;73:921. - PubMed
Beydoun 1986
-
- Beydoun S, Yasin S. Premature rupture of the membranes before 28 weeks: conservative management. American Journal of Obstetrics and Gynecology 1986;155:471. - PubMed
Borgida 2000
-
- Borgida AF, Mills AA, Feldman DM, Rodis JF, Egan JF. Outcome of pregnancies complicated by ruptured membranes after genetic amniocentesis. American Journal of Obstetrics and Gynecology 2000;183(4):937‐9. - PubMed
Bryant‐Greenwood 1998
-
- Bryant‐Greenwood GD. The extracellular matrix of the human fetal membranes: structure and function. Placenta 1998;19(1):1‐11. - PubMed
Cobo 2007
-
- Cobo T, Borrell A, Fortuny A. Treatment with Amniopatch of premature rupture of the membranes after first trimester chorionic villus sampling. Prenatal Diagnosis 2007;27:1024‐7. - PubMed
Cotton 1984
-
- Cotton DB, Hill LM, Strassner HT, Platt LD, Ledger WJ. Use of amniocentesis in preterm gestation with ruptured membranes. Obstetrics and Gynecology 1984;63:38‐48. - PubMed
Deprest 2006
-
- Deprest J, Jani J, Lewi L, Ochsenbein‐Kölble N, Cannie M, Doné E, et al. Fetoscopic surgery: encouraged by clinical experience and boosted by instrument innovation. Seminars in Fetal and Neonatal Medicine 2006;11(6):398‐412. - PubMed
Deprest 2011
-
- Deprest J, Emonds MP, Richter J, DeKoninck P, Mieghem T, Schoubroeck D, et al. Amniopatch for iatrogenic rupture of the fetal membranes. Prenatal Diagnosis 2011;31(7):661‐6. - PubMed
Devlieger 2009
-
- Devlieger R, Verhaeghe J, Coopmans W, Deprest JA. IGFBP‐1 levels in cervicovaginal secretions before and after amniocentesis. Gynecologic and Obstetric Investigation 2009;67(1):9‐13. - PubMed
Gomez 1998
-
- Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. American Journal of Obstetrics and Gynecology 1998;179(1):194‐202. - PubMed
Gratacos 2006
-
- Gratacos E, Sanin‐Blair J, Lewi L, Toran N, Verbist G, Cabero L. A histological study of fetoscopic membrane defects to document membrane healing. Placenta 2006;27:452‐6. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lavery 1982
-
- Lavery JP, Miller CE, Knight RD. The effect of labor on the rheologic response of chorioamniotic membranes. Obstetrics and Gynecology 1982;60(1):87‐92. - PubMed
Mercer 2003
-
- Mercer BM. Preterm premature rupture of the membranes. Obstetrics and Gynecology 2003;101:178‐93. - PubMed
O'Brien 2006
-
- O’Brien JM, Milligan DA, Barton JR. Transcervical intervention for previable premature rupture of the membranes. American Journal of Obstetrics and Gynecology 2006;195:S68.
Qin 1997
-
- Qin X, Garibay‐Tupas J, Chua PK, Cachola L, Bryant‐Greenwood GD. An autocrine/paracrine role of human decidual relaxin. I. Interstitial collagenase (matrix metalloproteinase‐1) and tissue plasminogen activator. Biology of Reproduction 1997;56(4):800‐11. - PubMed
Quintero 1999
-
- Quintero RA, Morales WJ, Allen M, Bornick PW, Arroyo J, LeParc G. Treatment of iatrogenic previable premature rupture of membranes with intra‐amniotic injection of platelets and cryoprecipitate (amniopatch): preliminary experience. American Journal of Obstetrics and Gynecology 1999;181(3):744‐9. - PubMed
RCOG 2006
-
- Royal College of Obstetricians and Gynaecologists. Preterm Prelabour Rupture of Membranes. RCOG Guidelines No 44. RCOG, November 2006.
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sciscione 2001
-
- Sciscione AC, Manley JA, Pollock M, Maas B, Schlossman PA, Mulla W, et al. Intracervical fibrin sealants: a potential treatment for early preterm premature rupture of the membranes. American Journal of Obstetrics and Gynecology 2001;184(3):368‐73. - PubMed
Tabor 2010
-
- Tabor A, Alfirevic Z. Update on procedure‐related risks for prenatal diagnosis techniques. Fetal Diagnosis and Therapy 2010;27:1‐7. - PubMed
Wideman 1964
-
- Wideman GL, Baird GH, Bolding OT. Ascorbic acid deficiency and premature rupture of the fetal membranes. American Journal of Obstetrics and Gynecology 1964;88:592‐5. - PubMed
Yoon 2003
-
- Yoon BH, Park CW, Chaiworapongsa T. Intrauterine infection and the development of cerebral palsy. BJOG: an international journal of obstetrics and gynaecology 2003;110(Suppl 20):124‐7. - PubMed
Young 2000
-
- Young BK, Roque H, Abdelhak A. Minimally invasive endoscopy in the treatment of preterm premature rupture of the membranes by application of fibrin sealant. Journal of Perinatal Medicine 2000;28:326‐30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

