miR-7a/b attenuates post-myocardial infarction remodeling and protects H9c2 cardiomyoblast against hypoxia-induced apoptosis involving Sp1 and PARP-1
- PMID: 27384152
- PMCID: PMC4935883
- DOI: 10.1038/srep29082
miR-7a/b attenuates post-myocardial infarction remodeling and protects H9c2 cardiomyoblast against hypoxia-induced apoptosis involving Sp1 and PARP-1
Abstract
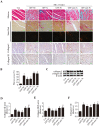
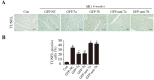
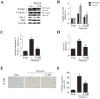
miRs (microRNAs, miRNAs) intricately regulate physiological and pathological processes. Although miR-7a/b protects against cardiomyocyte injury in ischemia/reperfusion injury, the function of miR-7a/b in myocardial infarction (MI)-induced cardiac remodeling remains unclear. Here, we sought to investigate the function of miR-7a/b in post-MI remodeling in a mouse model and to determine the underlying mechanisms involved. miR-7a/b overexpression improved cardiac function, attenuated cardiac remodeling and reduced fibrosis and apoptosis, whereas miR-7a/b silencing caused the opposite effects. Furthermore, miR-7a/b overexpression suppressed specific protein 1 (Sp1) and poly (ADP-ribose) polymerase (PARP-1) expression both in vivo and in vitro, and a luciferase reporter activity assay showed that miR-7a/b could directly bind to Sp1. Mithramycin, an inhibitor of the DNA binding activity of Sp1, effectively repressed PARP-1 and caspase-3, whereas knocking down miR-7a/b partially counteracted these beneficial effects. Additionally, an immunoprecipitation assay indicated that hypoxia triggered activation of the binding activity of Sp1 to the promoters of PARP-1 and caspase-3, which is abrogated by miR-7a/b. In summary, these findings identified miR-7a/b as protectors of cardiac remodeling and hypoxia-induced injury in H9c2 cardiomyoblasts involving Sp1 and PARP-1.
Figures




Similar articles
-
The Circular RNA Cdr1as Promotes Myocardial Infarction by Mediating the Regulation of miR-7a on Its Target Genes Expression.PLoS One. 2016 Mar 21;11(3):e0151753. doi: 10.1371/journal.pone.0151753. eCollection 2016. PLoS One. 2016. PMID: 26998750 Free PMC article.
-
MicroRNA-223 protects neonatal rat cardiomyocytes and H9c2 cells from hypoxia-induced apoptosis and excessive autophagy via the Akt/mTOR pathway by targeting PARP-1.J Mol Cell Cardiol. 2018 May;118:133-146. doi: 10.1016/j.yjmcc.2018.03.018. Epub 2018 Mar 31. J Mol Cell Cardiol. 2018. PMID: 29608885
-
Curcumin protects cardiac myocyte against hypoxia-induced apoptosis through upregulating miR-7a/b expression.Biomed Pharmacother. 2016 Jul;81:258-264. doi: 10.1016/j.biopha.2016.04.020. Epub 2016 Apr 21. Biomed Pharmacother. 2016. PMID: 27261602
-
Urocortin Role in Ischemia Cardioprotection and the Adverse Cardiac Remodeling.Int J Mol Sci. 2021 Nov 9;22(22):12115. doi: 10.3390/ijms222212115. Int J Mol Sci. 2021. PMID: 34829997 Free PMC article. Review.
-
The microRNA in ventricular remodeling: the miR-30 family.Biosci Rep. 2019 Aug 2;39(8):BSR20190788. doi: 10.1042/BSR20190788. Print 2019 Aug 30. Biosci Rep. 2019. PMID: 31320543 Free PMC article. Review.
Cited by
-
EPC-Derived Exosomal miR-1246 and miR-1290 Regulate Phenotypic Changes of Fibroblasts to Endothelial Cells to Exert Protective Effects on Myocardial Infarction by Targeting ELF5 and SP1.Front Cell Dev Biol. 2021 May 13;9:647763. doi: 10.3389/fcell.2021.647763. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34055778 Free PMC article.
-
Naringin attenuates angiotensin II induced cardiac hypertrophy by inhibiting carbonic anhydrase II.Sci Rep. 2025 Apr 6;15(1):11789. doi: 10.1038/s41598-025-95537-2. Sci Rep. 2025. PMID: 40189613 Free PMC article.
-
MMP-12 polarizes neutrophil signalome towards an apoptotic signature.J Proteomics. 2022 Jul 30;264:104636. doi: 10.1016/j.jprot.2022.104636. Epub 2022 Jun 2. J Proteomics. 2022. PMID: 35661763 Free PMC article.
-
Sp1 Plays an Important Role in Vascular Calcification Both In Vivo and In Vitro.J Am Heart Assoc. 2018 Mar 23;7(6):e007555. doi: 10.1161/JAHA.117.007555. J Am Heart Assoc. 2018. PMID: 29572322 Free PMC article.
-
Specific protein 1 inhibitor mithramycin A protects cardiomyocytes from myocardial infarction via interacting with PARP.In Vitro Cell Dev Biol Anim. 2021 Mar;57(3):315-323. doi: 10.1007/s11626-021-00543-z. Epub 2021 Feb 12. In Vitro Cell Dev Biol Anim. 2021. PMID: 33580416
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

