Aldehyde dehydrogenase inhibition combined with phenformin treatment reversed NSCLC through ATP depletion
- PMID: 27384481
- PMCID: PMC5226516
- DOI: 10.18632/oncotarget.10354
Aldehyde dehydrogenase inhibition combined with phenformin treatment reversed NSCLC through ATP depletion
Abstract
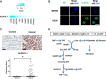
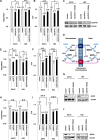
Among ALDH isoforms, ALDH1L1 in the folate pathway showed highly increased expression in non-small-cell lung cancer cells (NSCLC). Based on the basic mechanism of ALDH converting aldehyde to carboxylic acid with by-product NADH, we suggested that ALDH1L1 may contribute to ATP production using NADH through oxidative phosphorylation. ALDH1L1 knockdown reduced ATP production by up to 60% concomitantly with decrease of NADH in NSCLC. ALDH inhibitor, gossypol, also reduced ATP production in a dose dependent manner together with decrease of NADH level in NSCLC. A combination treatment of gossypol with phenformin, mitochondrial complex I inhibitor, synergized ATP depletion, which efficiently induced cell death. Pre-clinical xenograft model using human NSCLC demonstrated a remarkable therapeutic response to the combined treatment of gossypol and phenformin.
Keywords: NSCLC; aldehyde dehydrogenase; cancer metabolism; gossypol; phenformin.
Conflict of interest statement
We confirm that the authors have no competing financial interests to declare.
Figures





References
-
- Wong DW, Leung EL, So KK, Tam IY, Sihoe AD, Cheng LC, Ho KK, Au JS, Chung LP, Pik Wong M, University of Hong Kong Lung Cancer Study G. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer. 2009;115:1723–1733. - PubMed
-
- Okamoto H, Watanabe K, Kunikane H, Yokoyama A, Kudoh S, Asakawa T, Shibata T, Kunitoh H, Tamura T, Saijo N. Randomised phase III trial of carboplatin plus etoposide vs split doses of cisplatin plus etoposide in elderly or poor-risk patients with extensive disease small-cell lung cancer: JCOG 9702. Br J Cancer. 2007;97:162–169. - PMC - PubMed
-
- Schmittel A, Fischer von Weikersthal L, Sebastian M, Martus P, Schulze K, Hortig P, Reeb M, Thiel E, Keilholz U. A randomized phase II trial of irinotecan plus carboplatin versus etoposide plus carboplatin treatment in patients with extended disease small-cell lung cancer. Ann Oncol. 2006;17:663–667. - PubMed
-
- Li X, Wan L, Geng J, Wu CL, Bai X. Aldehyde dehydrogenase 1A1 possesses stem-like properties and predicts lung cancer patient outcome. J Thorac Oncol. 2012;7:1235–1245. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

