A mouse model for testing remyelinating therapies
- PMID: 27384502
- PMCID: PMC5207347
- DOI: 10.1016/j.expneurol.2016.06.033
A mouse model for testing remyelinating therapies
Abstract
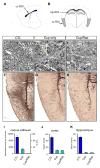
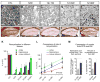
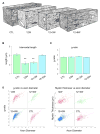
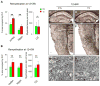
Used in combination with immunomodulatory therapies, remyelinating therapies are a viable therapeutic approach for treating individuals with multiple sclerosis. Studies of postmortem MS brains identified greater remyelination in demyelinated cerebral cortex than in demyelinated brain white matter and implicated reactive astrocytes as an inhibitor of white matter remyelination. An animal model that recapitulates these phenotypes would benefit the development of remyelination therapeutics. We have used a modified cuprizone protocol that causes a consistent and robust demyelination of mouse white matter and cerebral cortex. Spontaneous remyelination occurred significantly faster in the cerebral cortex than in white matter and reactive astrocytes were more abundant in white matter lesions. Remyelination of white matter and cerebral cortex was therapeutically enhanced by daily injections of thyroid hormone triiodothyronine (T3). In summary, we describe an in vivo demyelination/remyelination paradigm that can be powered to determine efficacy of therapies that enhance white matter and cortical remyelination.
Keywords: Differential remyelination; Internodal length; Mice; Myelin; Therapeutic; Ultrastructure.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

References
-
- Baas D, Bourbeau D, Sarliève LL, Ittel ME, Dussault JH, Puymirat J. Oligodendrocyte maturation and progenitor cell proliferation are independently regulated by thyroid hormone. Glia. 1997;19:324–332. - PubMed
-
- Back SA, Tuohy TMF, Chen H, Wallingford N, Craig A, Struve J, Luo NL, Banine F, Liu Y, Chang A, Trapp BD. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11:966–972. - PubMed
-
- Blakemore WF. Remyelination of the superior cerebellar peduncle in old mice following demyelination induced by cuprizone. J Neurol Sci. 1974;22:121–126. - PubMed
-
- Blakemore WF, Franklin RJM. Remyelination in experimental models of toxin-induced demyelination. Curr Top Microbiol Immunol. 2008;318:193–212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

