Healthcare system-wide implementation of opioid-safety guideline recommendations: the case of urine drug screening and opioid-patient suicide- and overdose-related events in the Veterans Health Administration
- PMID: 27384953
- PMCID: PMC5110498
- DOI: 10.1007/s13142-016-0423-7
Healthcare system-wide implementation of opioid-safety guideline recommendations: the case of urine drug screening and opioid-patient suicide- and overdose-related events in the Veterans Health Administration
Abstract
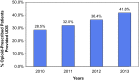
This study provides an example of how healthcare system-wide progress in implementation of opioid-therapy guideline recommendations can be longitudinally assessed and then related to subsequent opioid-prescribed patient health and safety outcomes. Using longitudinal linear mixed effects analyses, we determined that in the Department of Veterans Affairs (VA) healthcare system (n = 141 facilities), over the 4-year interval from 2010 to 2013, a key opioid therapy guideline recommendation, urine drug screening (UDS), increased from 29 to 42 %, with an average within-facility increase rate of 4.5 % per year. Higher levels of UDS implementation from 2010 to 2013 were associated with lower risk of suicide and drug overdose events among VA opioid-prescribed patients in 2013, even after adjusting for patients' 2012 demographic characteristics and medical and mental health comorbidities. Findings suggest that VA clinicians and healthcare policymakers have been responsive to the 2010 VA/Department of Defense (DOD) UDS treatment guideline recommendation, resulting in improved patient safety for VA opioid-prescribed patients.
Keywords: Drug overdose; Opioid therapy safety; Suicide; Treatment guideline implementation; Urine drug screening.
Conflict of interest statement
Compliance with ethical standards Research reported here was supported in part by the Department of Veterans Affairs Health Services Research and Development (HSR&D) grant number RRP 10-106. All work performed in this investigation was in accordance with the ethical standards of our institutional research committees and with the 1964 Helsinki Declaration and its later amendments. For this type of study, formal consent is not required. This research was approved by the Stanford University administrative panels for the protection of human subjects. Conflict of Interest The authors declare that they have no conflicts of interest.
Figures
References
-
- Centers for Disease Control and Prevention CDC grand rounds: prescription drug overdoses—A U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61:10–13. - PubMed
-
- Substance Abuse and Mental Health Service Administration, Office of Applied Studies. Drug Abuse Warning Network, 2007: National Estimates of Drug-Related Emergency Department Visits. Rockville, MD; 2010.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical