Dragon (RGMb) induces oxaliplatin resistance in colon cancer cells
- PMID: 27384995
- PMCID: PMC5216997
- DOI: 10.18632/oncotarget.10338
Dragon (RGMb) induces oxaliplatin resistance in colon cancer cells
Abstract
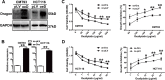
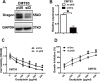
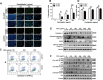
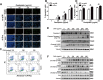
Colorectal cancer (CRC) is one of the most commonly diagnosed cancers and a major cause of cancer mortality. Chemotherapy resistance remains a major challenge for treating advanced CRC. Therefore, the identification of targets that induce drug resistance is a priority for the development of novel agents to overcome resistance. Dragon (also known as RGMb) is a member of the repulsive guidance molecule (RGM) family. We previously showed that Dragon expression increases with CRC progression in human patients. In the present study, we found that Dragon inhibited apoptosis and increased viability of CMT93 and HCT116 cells in the presence of oxaliplatin. Dragon induced resistance of xenograft tumor to oxaliplatinin treatment in mice. Mechanistically, Dragon inhibited oxaliplatin-induced JNK and p38 MAPK activation, and caspase-3 and PARP cleavages. Our results indicate that Dragon may be a novel target that induces drug resistance in CRC.
Keywords: JNK; colon cancer; oxaliplatin resistance; p38 MAPK; Dragon.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, Jemal A, Cho H, Anderson RN, Kohler BA, Eheman CR, Ward EM. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290–1314. - PMC - PubMed
-
- Mattick JS, Makunin Non-coding RNA. Hum Mol Genet. 2006;15(Spec No 1):R17–29. - PubMed
-
- Stec R, Bodnar L, Charkiewicz R, Korniluk J, Rokita M, Smoter M, Ciechowicz M, Chyczewski L, Niklinski J, Kozlowski W, Szczylik C. K-Ras gene mutation status as a prognostic and predictive factor in patients with colorectal cancer undergoing irinotecan- or oxaliplatin-based chemotherapy. Cancer Biol Ther. 2012;13:1235–1243. - PMC - PubMed
-
- Curia MC, De Iure S, De Lellis L, Veschi S, Mammarella S, White MJ, Bartlett J, Di Iorio A, Amatetti C, Lombardo M, Di Gregorio P, Battista P, Mariani-Costantini R, et al. Increased variance in germline allele-specific expression of APC associates with colorectal cancer. Gastroenterology. 2012;142:71–77. e71. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

