Leapfrogging: primordial germ cell transplantation permits recovery of CRISPR/Cas9-induced mutations in essential genes
- PMID: 27385011
- PMCID: PMC5004912
- DOI: 10.1242/dev.138057
Leapfrogging: primordial germ cell transplantation permits recovery of CRISPR/Cas9-induced mutations in essential genes
Abstract
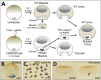
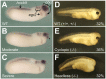
CRISPR/Cas9 genome editing is revolutionizing genetic loss-of-function analysis but technical limitations remain that slow progress when creating mutant lines. First, in conventional genetic breeding schemes, mosaic founder animals carrying mutant alleles are outcrossed to produce F1 heterozygotes. Phenotypic analysis occurs in the F2 generation following F1 intercrosses. Thus, mutant analyses will require multi-generational studies. Second, when targeting essential genes, efficient mutagenesis of founders is often lethal, preventing the acquisition of mature animals. Reducing mutagenesis levels may improve founder survival, but results in lower, more variable rates of germline transmission. Therefore, an efficient approach to study lethal mutations would be useful. To overcome these shortfalls, we introduce 'leapfrogging', a method combining efficient CRISPR mutagenesis with transplantation of mutated primordial germ cells into a wild-type host. Tested using Xenopus tropicalis, we show that founders containing transplants transmit mutant alleles with high efficiency. F1 offspring from intercrosses between F0 animals that carry embryonic lethal alleles recapitulate loss-of-function phenotypes, circumventing an entire generation of breeding. We anticipate that leapfrogging will be transferable to other species.
Keywords: CRISPR/Cas9; Genome editing; Knockouts; Primordial germ cells; TALENs; Xenopus.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures


References
-
- Ayadi A., Birling M.-C., Bottomley J., Bussell J., Fuchs H., Fray M., Gailus-Durner V., Greenaway S., Houghton R., Karp N. et al. (2012). Mouse large-scale phenotyping initiatives: overview of the European Mouse Disease Clinic (EUMODIC) and of the Wellcome Trust Sanger Institute Mouse Genetics Project. Mamm. Genome 23, 600-610. 10.1007/s00335-012-9418-y - DOI - PMC - PubMed
-
- Blackler A. W. and Fischberg M. (1961). Transfer of primordial germ-cells in Xenopus laevis. J. Embryol. Exp. Morph. 9, 634-641. - PubMed
-
- Blitz I. L. and Cho K. W. Y. (1995). Anterior neurectoderm is progressively induced during gastrulation: the role of the Xenopus homeobox gene orthodenticle. Development 121, 993-1004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

