The relationship between sex change and reproductive success in a protandric marine gastropod
- PMID: 27385040
- PMCID: PMC4935951
- DOI: 10.1038/srep29439
The relationship between sex change and reproductive success in a protandric marine gastropod
Abstract
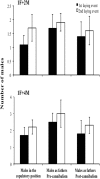
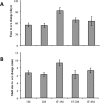
Protandric species switch sex during their lifetime. According to theory, the time (body size) at which sex change occurs is determined by the reproductive success of individuals affected by social interactions as well as by post-copulatory factors. Experimental evidence is biased to few social systems making the exploration of general patterns difficult. We used the protandric marine gastropod Crepidula coquimbensis that partakes in intrabrood sibling cannibalism to test the following hypotheses: 1. Male-male competition for access to females and sibling cannibalism determine male reproductive success; 2. Males with greater access to females and with higher reproductive success will have reduced growth rates and will delay sex change. Artificial aggregations with different social structures were constructed and male reproductive success was estimated by paternity analysis. The results supported our expectations showing that male competitive ability for access to the female, time spent by males in the copulatory position, and sibling cannibalism affect reproductive success and influence time to sex change, with less successful males hastening sex change. Also, males that spent more time in the copulatory position had reduced growth rates. Comparing these results with those reported for other sequential hermaphrodites provides evidence supporting general patterns of sex change in nature.
Figures





References
-
- Polikansky D. Sex change in plants and animals. Annu. Rev. Ecol. Syst. 13, 471–495 (1982).
-
- Charnov E. L. The Theory of Sex Allocation, Princeton University Press (1982). - PubMed
-
- Warner R. R., Robertson D. R. & Leigh E. G. Jr. Sex change and sexual selection. Science 190, 633–638 (1975). - PubMed
-
- Hoffman S. G., Schildhauer M. P. & Warner R. R. The costs of changing sex and the ontogeny of males under contest competition for mates. Evolution 39, 915–927 (1985). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

