Co-expression modules of NF1, PTEN and sprouty enable distinction of adult diffuse gliomas according to pathway activities of receptor tyrosine kinases
- PMID: 27385209
- PMCID: PMC5312298
- DOI: 10.18632/oncotarget.10359
Co-expression modules of NF1, PTEN and sprouty enable distinction of adult diffuse gliomas according to pathway activities of receptor tyrosine kinases
Abstract
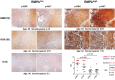
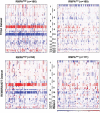
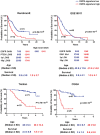
Inter-individual variability causing elevated signaling of receptor tyrosine kinases (RTK) may have hampered the efficacy of targeted therapies. We developed a molecular signature for clustering adult diffuse gliomas based on the extent of RTK pathway activities. Glioma gene modules co-expressed with NF1 (NF1-M), Sprouty (SPRY-M) and PTEN (PTEN-M) were identified, their signatures enabled robust clustering of adult diffuse gliomas of WHO grades II-IV from five independent data sets into two subtypes with distinct activities of RAS-RAF-MEK-MAPK cascade and PI3K-AKT pathway (named RMPAhigh and RMPAlow subtypes) in a morphology-independent manner. The RMPAhigh gliomas were associated with poor prognosis compared to the RMPAlow gliomas. The RMPAhigh and RMPAlow glioma subtypes harbored unique sets of genomic alterations in the RTK signaling-related genes. The RMPAhigh gliomas were enriched in immature vessel cells and tumor associated macrophages, and both cell types expressed high levels of pro-angiogenic RTKs including MET, VEGFR1, KDR, EPHB4 and NRP1. In gliomas with major genomic lesions unrelated to RTK pathway, high RMPA signature was associated with short survival. Thus, the RMPA signatures capture RTK activities in both glioma cells and glioma microenvironment, and RTK signaling in the glioma microenvironment contributes to glioma progression.
Keywords: glioma; molecular classification; receptor tyrosine kinase.
Conflict of interest statement
None of the authors have any competing interests.
Figures



Similar articles
-
IGFBP2 expression predicts IDH-mutant glioma patient survival.Oncotarget. 2017 Jan 3;8(1):191-202. doi: 10.18632/oncotarget.13329. Oncotarget. 2017. PMID: 27852048 Free PMC article.
-
Alterations in the RTK/Ras/PI3K/AKT pathway serve as potential biomarkers for immunotherapy outcome of diffuse gliomas.Aging (Albany NY). 2021 Jun 8;13(11):15444-15458. doi: 10.18632/aging.203102. Epub 2021 Jun 8. Aging (Albany NY). 2021. PMID: 34100771 Free PMC article.
-
Interactions between PTEN and receptor tyrosine kinase pathways and their implications for glioma therapy.Expert Rev Anticancer Ther. 2009 Feb;9(2):235-45. doi: 10.1586/14737140.9.2.235. Expert Rev Anticancer Ther. 2009. PMID: 19192961 Free PMC article. Review.
-
Pten signaling in gliomas.Neuro Oncol. 2002 Jul;4(3):196-211. Neuro Oncol. 2002. PMID: 12084351 Free PMC article. Review.
-
Akt- or MEK-mediated mTOR inhibition suppresses Nf1 optic glioma growth.Neuro Oncol. 2015 Jun;17(6):843-53. doi: 10.1093/neuonc/nou329. Epub 2014 Dec 21. Neuro Oncol. 2015. PMID: 25534823 Free PMC article.
Cited by
-
Simultaneous Knockdown of Sprouty2 and PTEN Promotes Axon Elongation of Adult Sensory Neurons.Front Cell Neurosci. 2020 Jan 21;13:583. doi: 10.3389/fncel.2019.00583. eCollection 2019. Front Cell Neurosci. 2020. PMID: 32038175 Free PMC article.
-
High FREM2 Gene and Protein Expression Are Associated with Favorable Prognosis of IDH-WT Glioblastomas.Cancers (Basel). 2019 Jul 27;11(8):1060. doi: 10.3390/cancers11081060. Cancers (Basel). 2019. PMID: 31357584 Free PMC article.
-
Sprouty2-a Novel Therapeutic Target in the Nervous System?Mol Neurobiol. 2019 Jun;56(6):3897-3903. doi: 10.1007/s12035-018-1338-8. Epub 2018 Sep 17. Mol Neurobiol. 2019. PMID: 30225774 Free PMC article. Review.
-
Sprouty2 enhances the tumorigenic potential of glioblastoma cells.Neuro Oncol. 2018 Jul 5;20(8):1044-1054. doi: 10.1093/neuonc/noy028. Neuro Oncol. 2018. PMID: 29635363 Free PMC article.
-
Contributions of immune cell populations in the maintenance, progression, and therapeutic modalities of glioma.AIMS Allergy Immunol. 2018;2(1):24-44. doi: 10.3934/allergy.2018.1.24. Epub 2018 Mar 21. AIMS Allergy Immunol. 2018. PMID: 32914058 Free PMC article.
References
-
- Ricard D, Idbaih A, Ducray F, Lahutte M, Hoang-Xuan K. Delattre JY: Primary brain tumours in adults. Lancet. 2012;379:1984–1996. - PubMed
-
- Suzuki H, Aoki K, Chiba K, Sato Y, Shiozawa Y, Shiraishi Y, Shimamura T, Niida A, Motomura K, Ohka F, Yamamoto T, Tanahashi K, Ranjit M, et al. Mutational landscape and clonal architecture in grade II and III gliomas. Nat Genet. 2015;47:458–468. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

