Protein kinase A can block EphA2 receptor-mediated cell repulsion by increasing EphA2 S897 phosphorylation
- PMID: 27385333
- PMCID: PMC5007095
- DOI: 10.1091/mbc.E16-01-0048
Protein kinase A can block EphA2 receptor-mediated cell repulsion by increasing EphA2 S897 phosphorylation
Abstract
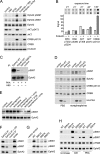
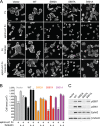
The EphA2 receptor tyrosine kinase plays key roles in tissue homeostasis and disease processes such as cancer, pathological angiogenesis, and inflammation through two distinct signaling mechanisms. EphA2 "canonical" signaling involves ephrin-A ligand binding, tyrosine autophosphorylation, and kinase activity; EphA2 "noncanonical" signaling involves phosphorylation of serine 897 (S897) by AKT and RSK kinases. To identify small molecules counteracting EphA2 canonical signaling, we developed a high-content screening platform measuring inhibition of ephrin-A1-induced PC3 prostate cancer cell retraction. Surprisingly, most hits from a screened collection of pharmacologically active compounds are agents that elevate intracellular cAMP by activating G protein-coupled receptors such as the β2-adrenoceptor. We found that cAMP promotes phosphorylation of S897 by protein kinase A (PKA) as well as increases the phosphorylation of several nearby serine/threonine residues, which constitute a phosphorylation hotspot. Whereas EphA2 canonical and noncanonical signaling have been viewed as mutually exclusive, we show that S897 phosphorylation by PKA can coexist with EphA2 tyrosine phosphorylation and block cell retraction induced by EphA2 kinase activity. Our findings reveal a novel paradigm in EphA2 function involving the interplay of canonical and noncanonical signaling and highlight the ability of the β2-adrenoceptor/cAMP/PKA axis to rewire EphA2 signaling in a subset of cancer cells.
© 2016 Barquilla et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures





References
-
- Astin JW, Batson J, Kadir S, Charlet J, Persad RA, Gillatt D, Oxley JD, Nobes CD. Competition amongst Eph receptors regulates contact inhibition of locomotion and invasiveness in prostate cancer cells. Nat Cell Biol. 2010;12:1194–1204. - PubMed
-
- Baell J, Walters MA. Chemistry: chemical con artists foil drug discovery. Nature. 2014;513:481–483. - PubMed
-
- Baell JB, Holloway GA. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J Med Chem. 2010;53:2719–2740. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

